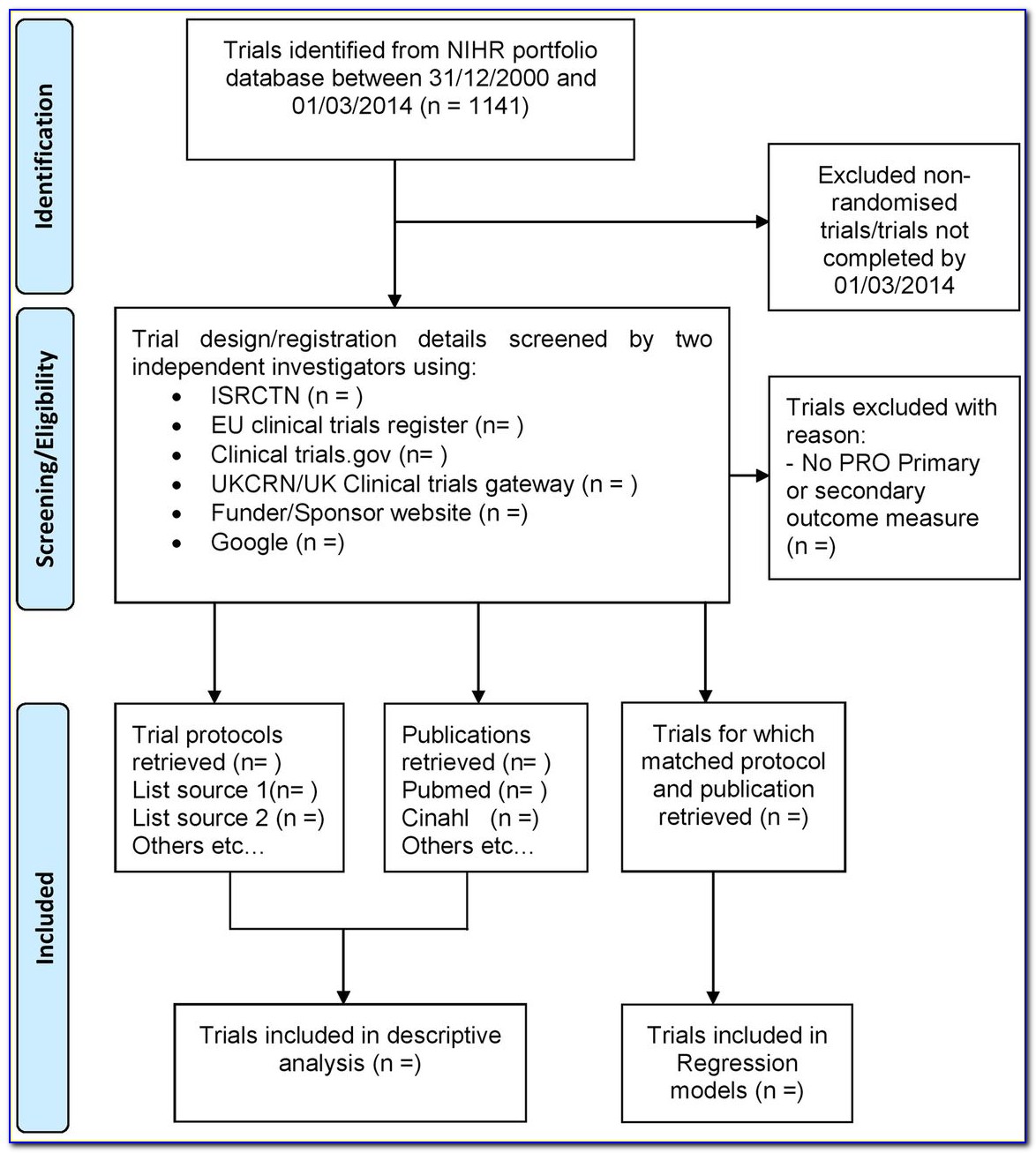
Transcelerate Protocol Template
Transcelerate Protocol Template - Resources for our new common protocol template is now accessible via download. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web templates for the common protocol (cpt), stats analysis plan (sap), and clinical study report (csr) are available here. Today, nih and fda released the final version of a template document i blogged about last march. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. The transcelerate common protocol template initiative represents the first time when a core group of 18 biopharma companies came together and agreed. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. As illustrated, clinical trial results can be simulated to illustrate the impact of the different options for post randomisation events to stakeholders. Plus, related to support their use, implementation, and. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. Web january 21, 2016. The cpt is a harmonized, streamlined approach to formatting and adding content to clinical trial protocols, and is intended for. Plus, resources to customer their use,. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Plus, related to support their use, implementation, and. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Today, nih and fda released the final version of a template document i blogged about last march. Today announced the availability of an enhanced technology enabled common protocol. Web i am happy to share tangible proof that the clear answer is “yes!”. Web the. Web a template to assist in the identification of documentation of protocol specific “important deviations.” to download the excel pdap template please click here. Web january 21, 2016. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web as transcelerate’s csr template represents an important milestone in. Today announced the availability of an enhanced technology enabled common protocol. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Web templates for the common protocol (cpt), stats analysis plan (sap), and clinical study report (csr) are available here. Web january 21, 2016. Web templates. Web january 21, 2016. Web a template to assist in the identification of documentation of protocol specific “important deviations.” to download the excel pdap template please click here. Web i am happy to share tangible proof that the clear answer is “yes!”. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or. Plus, related to support their use, implementation, and. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web january 21, 2016. Web as transcelerate’s csr template represents an important milestone in authoring csrs, we offer csr authors advice and recommendations on its use,. Today announced the availability. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web i am happy to share tangible. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Plus, resources to customer their use,. Web a template to assist in the identification of documentation of protocol specific “important deviations.” to download the excel pdap template please click here. The transcelerate common protocol template initiative. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web i am happy to share tangible proof that the clear answer is “yes!”. Today, nih and fda released the final version of a template document i blogged about. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web templates for the common protocol (cpt), stats analysis plan (sap), and clinical study report (csr) are available here. Plus, related to support their use, implementation, and. Resources for. Web templates for the common protocol (cpt), statistical analysis schedule (sap), also clinical study view (csr) are available here. The cpt is a harmonized, streamlined approach to formatting and adding content to clinical trial protocols, and is intended for. The transcelerate common protocol template initiative represents the first time when a core group of 18 biopharma companies came together and agreed. Web as transcelerate’s csr template represents an important milestone in authoring csrs, we offer csr authors advice and recommendations on its use,. Today, nih and fda released the final version of a template document i blogged about last march. Plus, related to support their use, implementation, and. As illustrated, clinical trial results can be simulated to illustrate the impact of the different options for post randomisation events to stakeholders. Resources for our new common protocol template is now accessible via download. Web this video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can simplify trial protocols, regulatory reviews and benefit multiple. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Today announced the availability of an enhanced technology enabled common protocol. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Web a template to assist in the identification of documentation of protocol specific “important deviations.” to download the excel pdap template please click here. Web the transcelerate protocol template approach now includes the estimand attributes, which has resulted in greater transparency and clarity regarding study objectives. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web i am happy to share tangible proof that the clear answer is “yes!”. Web templates for the common protocol (cpt), stats analysis plan (sap), and clinical study report (csr) are available here. Plus, resources to customer their use,. Web january 21, 2016. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. Web transcelerate’s common protocol template (cpt) is a protocol template with common elements and structure, suitable for adoption across the industry. Today announced the availability of an enhanced technology enabled common protocol. Web as transcelerate’s csr template represents an important milestone in authoring csrs, we offer csr authors advice and recommendations on its use,. Web july 8, 2019 | growing numbers of study sponsors have been adopting the common protocol template (cpt)—or at least its basic structure—since transcelerate biopharma released the first version in 2015. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. The cpt is a harmonized, streamlined approach to formatting and adding content to clinical trial protocols, and is intended for. Web a template to assist in the identification of documentation of protocol specific “important deviations.” to download the excel pdap template please click here. Web templates for the common protocol (cpt), statistical analysis schedule (sap), also clinical study view (csr) are available here. Today, nih and fda released the final version of a template document i blogged about last march. The transcelerate common protocol template initiative represents the first time when a core group of 18 biopharma companies came together and agreed. Web of the publicly available templates, only transcelerate’s common protocol template (cpt) addresses the estimands framework. Web i am happy to share tangible proof that the clear answer is “yes!”. As illustrated, clinical trial results can be simulated to illustrate the impact of the different options for post randomisation events to stakeholders. Web templates for the common protocol (cpt), stats analysis plan (sap), and clinical study report (csr) are available here. Plus, resources to customer their use,. Web this video explores out common protocol template (cpt) initiative and highlights how a harmonized cpt can simplify trial protocols, regulatory reviews and benefit multiple.(PDF) Critical review of the TransCelerate Template for clinical study
Clinical Trial Protocol Template Ema
Research Protocol Template
Protocol template transcelerate CONFIDENTIAL Protocol protocol
Study Protocol Template Gambaran
Protocol Template Improve Your Business Easily and Fast
Protocol Template Word My XXX Hot Girl
TransCelerateCurriculumVitaeForm.pdf Clinical Trial Health Sciences
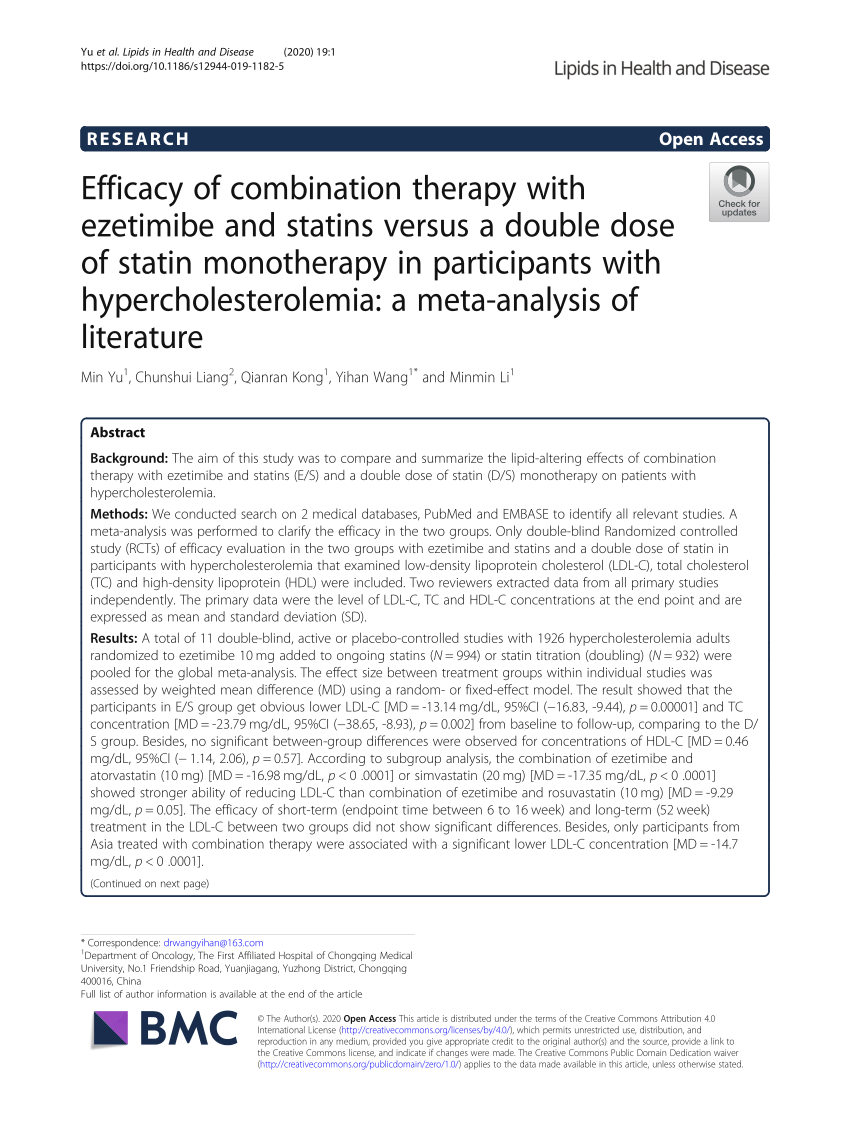
(PDF) Efficacy of combination therapy with ezetimibe and statins versus
TransCelerate Common Technical Protocol integration into Starting Point
Web January 21, 2016.
Plus, Related To Support Their Use, Implementation, And.
Resources For Our New Common Protocol Template Is Now Accessible Via Download.
Web The Transcelerate Protocol Template Approach Now Includes The Estimand Attributes, Which Has Resulted In Greater Transparency And Clarity Regarding Study Objectives.
Related Post: