Qms Procedure Template
Qms Procedure Template - The stages in this process are. Web mdsap qms f0002.2.003 new document proposal (ndp) template and guidelines mdsap qms p0004 mdsap qms p0004.003: It will also aid in. Web characteristics of qms internal audit procedure: Web download our 100% editable quality management system template to make analysis more accessible, faster, and accurate. The qms documentation can be represented as a hierarchy, as shown in the diagram below: Ad enhance your quality processes and cut costs with the #1 qms software. Better communication that lowers your manufacturing down time & improves your efficiencies Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other. Web four parts of the quality management plan template: Web a quality management system (qms) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and. Discover the ready to use, ai powered qms software. Better communication that lowers your manufacturing down time & improves your efficiencies The qms assists businesses in documenting their. This is a four stage process. Ad enhance your quality processes and cut costs with the #1 qms software. Web characteristics of qms internal audit procedure: The qms documentation can consist of different types of documents. This is a quality management cycle example diagram sample. How often should changes be made to keep up with industry best practices? Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other. The qm is divided into ten. The stages in this process are. Descriptions of the processes of the qms and their interaction. Web download our 100% editable quality management system template to make. A quality standard provides requirements for products and services. Ad no matter your mission, get the right qms systems to accomplish it. Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other. Covering a range of quality standards and roles. The qm is. Web quality management system (qms) stands for a standard set of protocols, procedures, and responsibilities that enhance the quality of your organization’s products. Web a quality management system is a formalized system. Web a quality management system (qms) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and. Discover the ready to use,. The qms assists businesses in documenting their. Web download our 100% editable quality management system template to make analysis more accessible, faster, and accurate. Better communication that lowers your manufacturing down time & improves your efficiencies Web features of these powerpoint presentation slides: We all have a standard template for our quality system procedures. Web characteristics of qms internal audit procedure: Easily find the qms systems you're looking for w/ our comparison grid. Web the oxebridge totally free iso 9001:2015 qms documentation template kit (or “otfiso90012015qmsdtk” for short) includes a full set of qms documentation. Web a quality management system is a formalized system. Discover the ready to use, ai powered qms software. Typically, we begin with purpose, scope, and definitions. Web the qm provides procedures or references for activities comprising the qms to ensure compliance to the necessary requirements of the standard. Web four parts of the quality management plan template: Train in as9100d, as13100, dprv, iso 13485, and more. Web the oxebridge totally free iso 9001:2015 qms documentation template kit (or. Train in as9100d, as13100, dprv, iso 13485, and more. Typically, we begin with purpose, scope, and definitions. This is a quality management cycle example diagram sample. The qms documentation can be represented as a hierarchy, as shown in the diagram below: Easily find the qms systems you're looking for w/ our comparison grid. Web download our 100% editable quality management system template to make analysis more accessible, faster, and accurate. Web characteristics of qms internal audit procedure: How often should changes be made to keep up with industry best practices? A quality standard provides requirements for products and services. Web four parts of the quality management plan template: Web a qms will incorporate documentation of processes, procedures, and responsibilities for achieving specific quality policies and objectives. Train in as9100d, as13100, dprv, iso 13485, and more. Descriptions of the processes of the qms and their interaction. Web what information should be included in the quality manual word template? Web the oxebridge totally free iso 9001:2015 qms documentation template kit (or “otfiso90012015qmsdtk” for short) includes a full set of qms documentation. Covering a range of quality standards and roles. Web quality management system (qms) stands for a standard set of protocols, procedures, and responsibilities that enhance the quality of your organization’s products. The qm is divided into ten. How often should changes be made to keep up with industry best practices? Qms internal audit procedure is a systematic and independent examination of financial and operational. The stages in this process are. The qms documentation can consist of different types of documents. Web characteristics of qms internal audit procedure: Easily find the qms systems you're looking for w/ our comparison grid. The qms documentation can be represented as a hierarchy, as shown in the diagram below: Ad ensure the quality of your products with a great user interface & visual critical alerts. Web a quality management system (qms) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and. Web qms 9001 document control procedure template is a simple to use, yet powerful document control procedure template that will help you effectively manage. This is a quality management cycle example diagram sample. Ad enhance your quality processes and cut costs with the #1 qms software. Web quality management system (qms) stands for a standard set of protocols, procedures, and responsibilities that enhance the quality of your organization’s products. This is a four stage process. It will also aid in. Web a quality management system is a formalized system. Web four parts of the quality management plan template: Web download our 100% editable quality management system template to make analysis more accessible, faster, and accurate. How often should changes be made to keep up with industry best practices? Easily find the qms systems you're looking for w/ our comparison grid. The qm is divided into ten. The qms documentation can be represented as a hierarchy, as shown in the diagram below: Covering a range of quality standards and roles. A quality standard provides requirements for products and services. Web features of these powerpoint presentation slides: Ad enhance your quality processes and cut costs with the #1 qms software. Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other. Descriptions of the processes of the qms and their interaction.QMS documentation Documentation Specification (Technical Standard)
QMS Manual.docx Quality Management Quality Management System Free
Example 2 QM 001 Quality Management System Scope Quality Management
Quality Manual Template Example Iso 9000 Quality Management System
Top 10 quality management system template in 2023 Chuyên Trang Chia
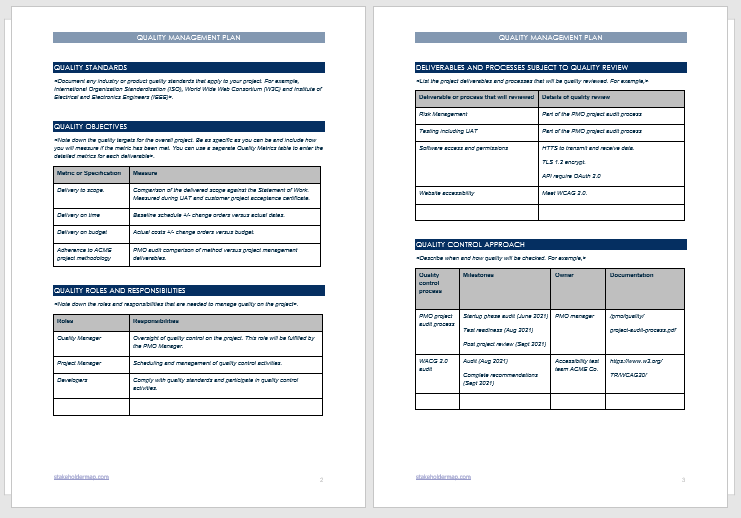
Free 11 Quality Management Plan Examples Pdf Word Examples Quality
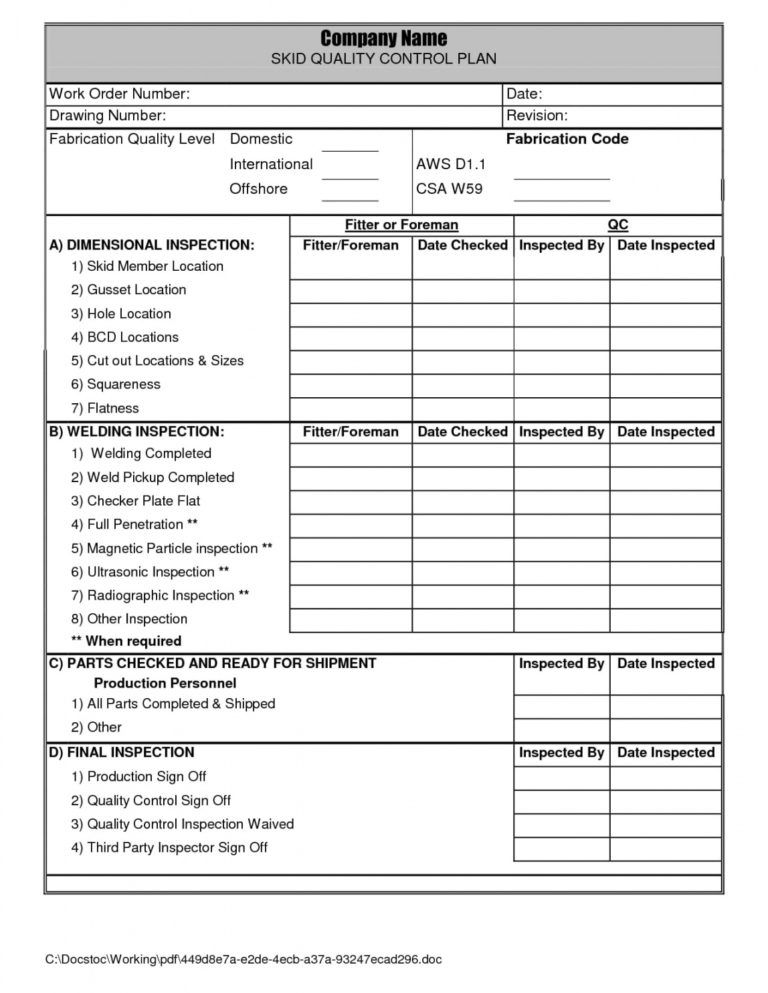
QMS065ManufacturingReworkProceduresample.pdf Quality Assurance
FREE 6+ Sample Management Review Templates in PDF MS Word
ISO 90012015 QMS Structure Infographic Flow chart, Process map
Quality Management Plan Sample Master of Template Document
Web Mdsap Qms F0002.2.003 New Document Proposal (Ndp) Template And Guidelines Mdsap Qms P0004 Mdsap Qms P0004.003:
We All Have A Standard Template For Our Quality System Procedures.
It Documents Processes, Procedures, And Responsibilities For Achieving Quality Policies As Well As Objectives.
Qms Internal Audit Procedure Is A Systematic And Independent Examination Of Financial And Operational.
Related Post: