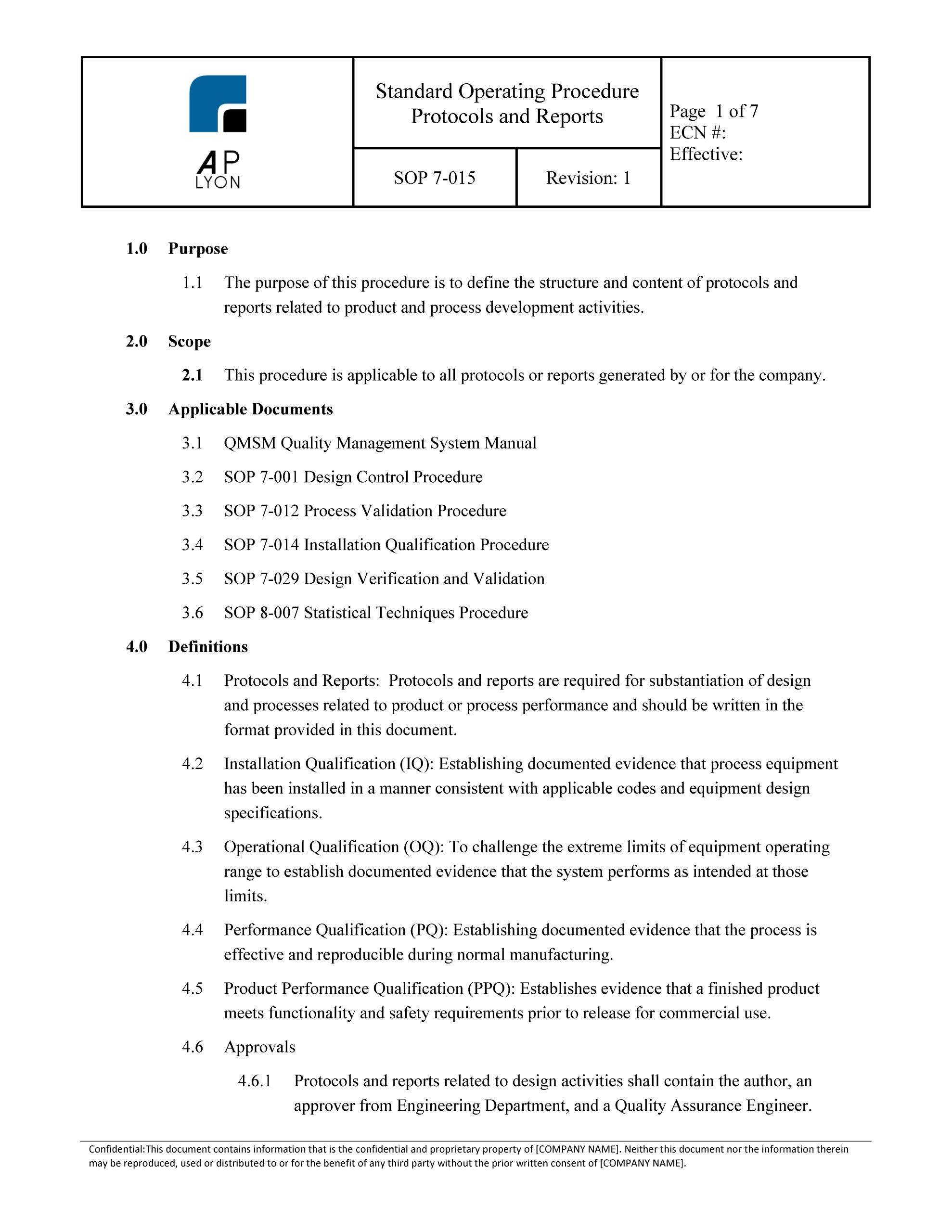
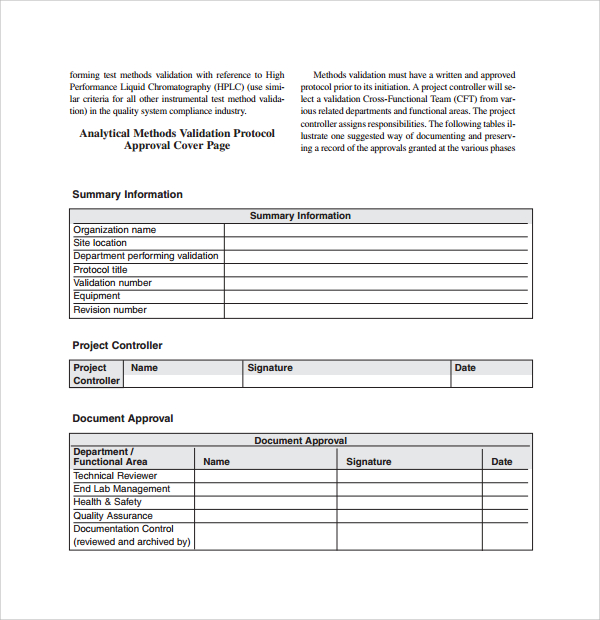
Medical Device Verification And Validation Plan Template
Medical Device Verification And Validation Plan Template - 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical device regulators forum. Any set of criteria can be subjected to verification. Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. ⇓ download this article as pdf. Did we make what we said we would make? We lack experience in this area and to see an example of how this should be done would be incredibly helpful. Define validation objectives and hypotheses, step 3: 21 cfr 820.30 design controls (f) design verification. As with other options, the files come in either word or excel format. Define equipment and processes to which these guidelines apply, step 2: It requires you to document each of these design outputs because they are evidence you met the design inputs. Validation 3.8.13 (bs en iso 9001:2015) Define validation objectives and hypotheses, step 3: Web this guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the validation of medical device software or the validation.. The plan should reference the applicable protocol and report for each item in the plan. Web quality system regulation process validation fda small business regulatory education for industry (redi) silver spring md september 30, 2015 joseph tartal Web verification and validation aspects of specified design envelope and medical device production system authoring group: Package consists of the procedure and a. Web verification is the process of ensuring your medical device satisfies the design inputs. The device is a basic stainless steel instrument. In our first post we covered the basics of process validation, and in subsequent posts we cover iq, oq, pq, and revalidation. Define validation objectives and hypotheses, step 3: We lack experience in this area and to see. At some point in the new medical device development, design verification must be performed to satisfy the applicable regulations and standards such as: Package consists of the procedure and a design review report form. Web verification is the process of ensuring your medical device satisfies the design inputs. As with other options, the files come in either word or excel. Web quality system regulation process validation fda small business regulatory education for industry (redi) silver spring md september 30, 2015 joseph tartal Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web the purpose of the start is until develop a plan available endorsement and. Web this guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the validation of medical device software or the validation. The device is a basic stainless steel instrument. We lack experience in this area and to see an example of how this should be done would be incredibly helpful. Execute necessary test. Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. All the equipment, processes, and software requiring validation should be included in the mvp. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. In our first post we. Trusted by leading pharma, biotech, and medical device companies globally. Download the entire series in one convenient pdf. Package consists of the procedure and a design review report form. Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. Web their complete medical device qms template package is available for $875, and it. Web verification is the process of ensuring your medical device satisfies the design inputs. It requires you to document each of these design outputs because they are evidence you met the design inputs. 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical device regulators forum. Web a master validation plan (mvp) is simply a. Web verification and validation aspects of specified design envelope and medical device production system authoring group: Download the entire series in one convenient pdf. We lack experience in this area and to see an example of how this should be done would be incredibly helpful. 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web 3.8.12 (bs en iso 9001:2015) confirmation, through the provision of objective evidence that specified requirements have been fulfilled. We lack experience in this area and to see an example of how this should be done would be incredibly helpful. Web verification and validation aspects of specified design envelope and medical device production system authoring group: ⇓ download this article as pdf. At some point in the new medical device development, design verification must be performed to satisfy the applicable regulations and standards such as: Trusted by leading pharma, biotech, and medical device companies globally. Download the entire series in one convenient pdf. Define validation objectives and hypotheses, step 3: Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web the purpose of the start is until develop a plan available endorsement and verification activities in the design and technology process. 21 cfr 820.30 design controls (f) design verification. Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: As an added bonus, med dev qms will refund the entire purchase price if you’re not 100%. Any set of criteria can be subjected to verification. Define equipment and processes to which these guidelines apply, step 2: Execute necessary test runs and record results, Validation 3.8.13 (bs en iso 9001:2015) Did we make what we said we would make? Web quality system regulation process validation fda small business regulatory education for industry (redi) silver spring md september 30, 2015 joseph tartal Web verification and validation aspects of specified design envelope and medical device production system authoring group: The device is a basic stainless steel instrument. The plan should reference the applicable protocol and report for each item in the plan. As an added bonus, med dev qms will refund the entire purchase price if you’re not 100%. Ad digitize and manage any validation, commissioning or qualification process. Package consists of the procedure and a design review report form. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Define validation objectives and hypotheses, step 3: ⇓ download this article as pdf. Web jun 20, 2019 #1 dear all, does anybody have a template or example for verification and validation activities and associated testing plan for 13485 (no software)? 21 cfr 820.30 design controls (f) design verification. Define equipment and processes to which these guidelines apply, step 2: Web 3.8.12 (bs en iso 9001:2015) confirmation, through the provision of objective evidence that specified requirements have been fulfilled. Web medical device design verification essentials. It requires you to document each of these design outputs because they are evidence you met the design inputs. Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities.Validation Protocols Reports Procedure
Verification and Validation Plan Template (MS Word) Templates, Forms
Medical Device Design Verification SOP
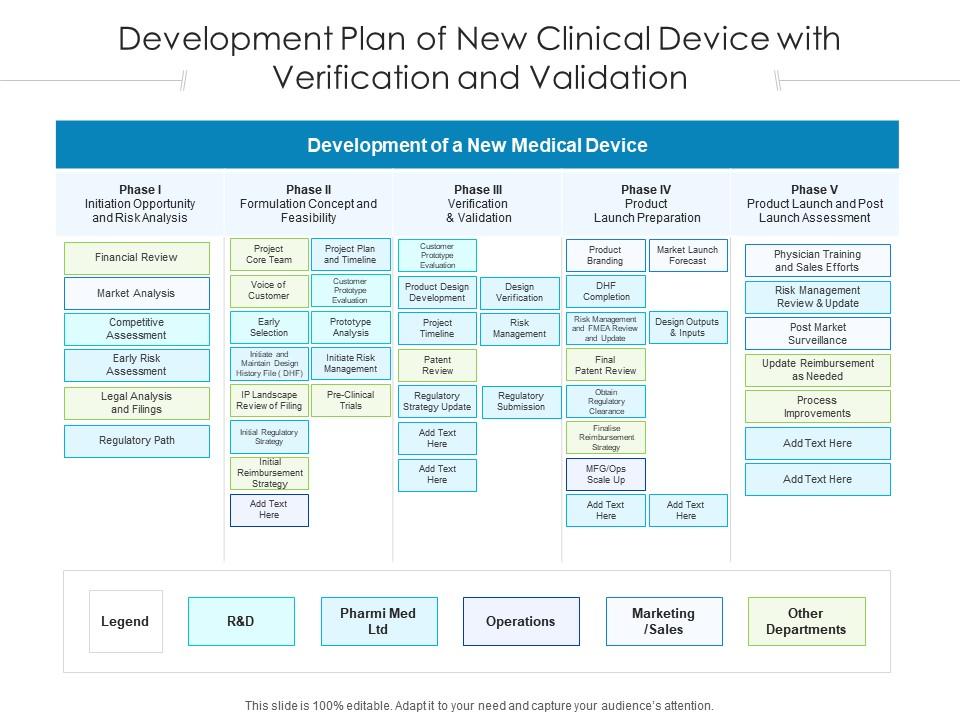
Development Plan Of New Clinical Device With Verification And
Template Word Master Software Validation Test Plan according to the
10+ Validation Plan Templates Sample Templates
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
Conducting Medical Device Verification and Validation Tests
Verification and Validation Plan Template (MS Word) Templates, Forms
Template of a validation plan. Download Scientific Diagram
Web The Purpose Of The Start Is Until Develop A Plan Available Endorsement And Verification Activities In The Design And Technology Process.
Web This Guidance Outlines General Validation Principles That The Food And Drug Administration (Fda) Considers To Be Applicable To The Validation Of Medical Device Software Or The Validation.
As With Other Options, The Files Come In Either Word Or Excel Format.
10 August 2022 Tracey Duffy, Imdrf Chair This Document Was Produced By The International Medical Device Regulators Forum.
Related Post: