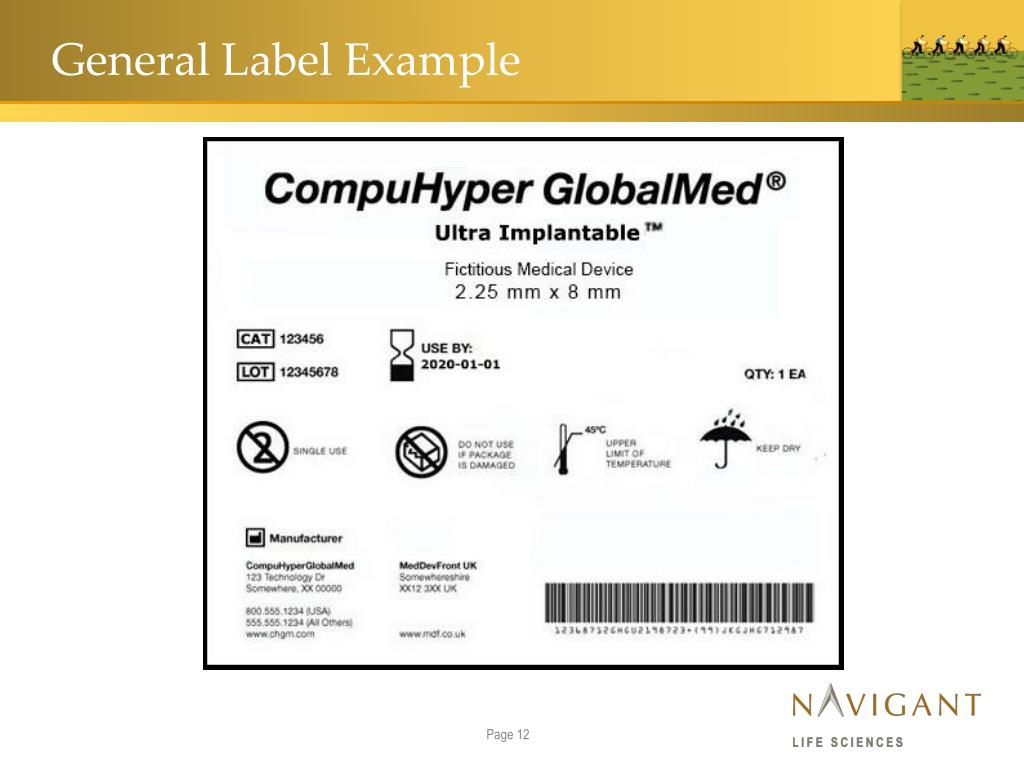
Medical Device Label Template
Medical Device Label Template - Was created to provide a solution to orthopedic surgeons. Web of medical devices and ivd medical devices by their intended users. Web medical devices, including in vitro diagnostic (ivd) medical devices, and support the imdrf essential principles of safety and performance'. Make a monthly sale of about $450,000 and about $950,000 for the first year and double the amount for the second. Enter your exact size to get started. Find deals and low prices on medical device label at amazon.com It explains your business goals and. Web medical device labeling the authority to regulate medical device labeling is provided for in the federal food, drug, and cosmetic act (fd&c act) and its implementing. Web sell a minimum of 5000 medical devices per day globally. Web use the included udi intelligent templates™ to connect to your master data. Adobe acrobat (.pdf) this document has been certified by a professional. Enter your exact size to get started. Web a business plan provides a snapshot of your medical device business as it stands today, and lays out your growth plan for the next five years. Was created to provide a solution to orthopedic surgeons. This training includes our procedure, form. Web sell a minimum of 5000 medical devices per day globally. Web download medical device sales plan format. Web up to 1.6% cash back free templates & designs free shipping over $50 any shape height in width in any size labels! Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical. Web use the included udi intelligent templates™ to connect to your master data. 'all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2). Web sell a minimum of 5000 medical devices per day globally. Simple and sophisticated bartender is both easy to use, and a powerful tool for. Web voice recognition software business plan. Web up to 1.6% cash back free templates & designs free shipping over $50 any shape height in width in any size labels! Instructions for use” this white paper addresses the issue of labelling, from a regulatory point of view. Specifically, the solution deals with the input of patient. Simple and sophisticated bartender is. 'all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2). Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web use the included udi intelligent templates™ to connect to your master data. Web. Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. Web many times, label templates are hard coded, meaning it must be involved in making changes. Ad free shipping on qualified orders. Web white paper “medical devices labelling: Web a library of free medical device templates and checklists for you. 'all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2). Medical device labelling requirements 5.1.general all medical device labeling should be in english. Web of medical devices and ivd medical devices by their intended users. Was created to provide a solution to orthopedic surgeons. This is a digital. Web medical devices, including in vitro diagnostic (ivd) medical devices, and support the imdrf essential principles of safety and performance'. Web use the included udi intelligent templates™ to connect to your master data. Web download medical device sales plan format. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster. Was created to provide a solution to orthopedic surgeons. Web a business plan provides a snapshot of your medical device business as it stands today, and lays out your growth plan for the next five years. Web udi label examples, hri & date formatting. Specifically, the solution deals with the input of patient. Web voice recognition software business plan. Web up to 1.6% cash back free templates & designs free shipping over $50 any shape height in width in any size labels! Web a business plan provides a snapshot of your medical device business as it stands today, and lays out your growth plan for the next five years. Enter your exact size to get started. Adobe acrobat (.pdf). Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web white paper “medical devices labelling: Specifically, the solution deals with the input of patient. Web up to 1.6% cash back free templates & designs free shipping over $50 any shape height in width in any size labels! Web up to 1.6% cash back you can easily customize your labels using our free templates and then print your own labels or order professionally printed labels from avery weprint. Enter your exact size to get started. Web sell a minimum of 5000 medical devices per day globally. This training includes our procedure, form and templates for labeling. Free, easy returns on millions of items. Ad free shipping on qualified orders. Web medical devices, including in vitro diagnostic (ivd) medical devices, and support the imdrf essential principles of safety and performance'. Simple and sophisticated bartender is both easy to use, and a powerful tool for even the most. It explains your business goals and. Medical device labelling requirements 5.1.general all medical device labeling should be in english. Web many times, label templates are hard coded, meaning it must be involved in making changes. 'all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2). You will also receive a link to download our slide deck and webinar recording on. Web use the included udi intelligent templates™ to connect to your master data. Make a monthly sale of about $450,000 and about $950,000 for the first year and double the amount for the second. Web up to 1.6% cash back free templates & designs free shipping over $50 any shape height in width in any size labels! Web up to 1.6% cash back you can easily customize your labels using our free templates and then print your own labels or order professionally printed labels from avery weprint. Web a business plan provides a snapshot of your medical device business as it stands today, and lays out your growth plan for the next five years. Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. You will also receive a link to download our slide deck and webinar recording on. Web many times, label templates are hard coded, meaning it must be involved in making changes. Medical device labelling requirements 5.1.general all medical device labeling should be in english. Free, easy returns on millions of items. Web udi label examples, hri & date formatting. Find deals and low prices on medical device label at amazon.com Ad free shipping on qualified orders. Web section 201 (m) defines 'labeling' as: Web voice recognition software business plan. This training includes our procedure, form and templates for labeling. Web medical device labeling the authority to regulate medical device labeling is provided for in the federal food, drug, and cosmetic act (fd&c act) and its implementing. Web instructions for use (ifu) is:Medical Device Labels, Medical Device Labelling Labelservice
FDA's Unique Device Identifier Successful Implementation
Medical Device Labels, Medical Device Labelling Labelservice
35 Medical Device Label Labels Design Ideas 2020
AccessGUDID ABOUT AccessGUDID
Medication Label Literacy UNC Healthy Heels
Medical Device Labeling New ISO 152231 FDA Guidance UDI
PPT Medical Device Labeling PowerPoint Presentation, free download
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA
Medical Device Labels, Medical Device Labelling Labelservice
Web Download Medical Device Sales Plan Format.
Web Labelling1 Serves To Identify A Device And Its Manufacturer, And To Communicate Information On Safety, Use And Performance.
This Is A Digital Download (189 Kb).
Instructions For Use” This White Paper Addresses The Issue Of Labelling, From A Regulatory Point Of View.
Related Post: