Master Validation Plan Template
Master Validation Plan Template - 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). Web up to 30% cash back the scope of this validation master plan is limited to the following: Appendices should contain all the relevant documentation referenced or stated in the. Company new buildings b, c product a, b, c existing building a. Want more free medical device resources? Web validation master plan examples. The following topics will be discussed in this validation master plan: Web here is a sample fda software validation template: Identify what needs to be validated. You can download a free sample of a validation master plan template in.pdf format. You can download a free sample of a validation master plan template in.pdf format. Define device and the validation approach. Identify what needs to be validated. Web the basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. You can create a great protocol, using a template. You can download a free sample of a validation master plan template in.pdf format. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance criteria, and documenting the necessary program that ensures a continuing. Web three (3) options the create a validation master plan. Web what is a. Identify what needs to be validated. 5.2.7 for large projects involving many materials, a materials validation plan may be used. Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Company new buildings b, c product a, b, c existing building a. Unlike a written plan for a specific validation project,. Want more free medical device resources? Select to establish a validation master map previous the next fda audit? You can create a great protocol, using a template. You canned creates one terrific protocol, through a template. Web what is validation master plan (vmp): Learn more & download a free sample here Web the validation master plan (vmp) is critical in achieving this goal by documenting compliance requirements and explaining necessary validation activities across a manufacturing operation. Systems, equipment, methods, facilities, etc., that are in the scope of the plan. With scribd, you can take your ebooks and audibooks anywhere, even offline. Web what. Web the basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. Web what is a validation master plan? • the scope and intended use of the facility • a. How in create a validation master plan before the next fda audit? 5.2.7 for large projects involving many materials,. To see the complete list of the most popular. Web the validation master plan (vmp) is critical in achieving this goal by documenting compliance requirements and explaining necessary validation activities across a manufacturing operation. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. • the scope. Want more free medical device resources? You can download a free sample of a validation master plan template in.pdf format. Appendices should contain all the relevant documentation referenced or stated in the. This document comprises individual recommendations on four topics relating to equipment qualification and process. Web a free master validation plan (mvp) form to help medical device manufacturers with. Web a medical device validation master plan (vmp) diagrams the standards associated with the capability of an office, characterizing the areas and systems to be approved, and gives a composed program for achieving and keeping up a certified. With scribd, you can take your ebooks and audibooks anywhere, even offline. Web three (3) options the create a validation master plan.. To see the complete list of the most popular. You sack download a free sample of a validation master plan document in.pdf format. Web up to 30% cash back the scope of this validation master plan is limited to the following: With scribd, you can take your ebooks and audibooks anywhere, even offline. • the scope and intended use of. Web a medical device validation master plan (vmp) diagrams the standards associated with the capability of an office, characterizing the areas and systems to be approved, and gives a composed program for achieving and keeping up a certified. Ad access millions of ebooks, audiobooks, podcasts, and more. Select to establish a validation master map previous the next fda audit? • the scope and intended use of the facility • a. Web validation master plan examples. This document comprises individual recommendations on four topics relating to equipment qualification and process. Teach additional & download a free sample here Define device and the validation approach. 5.2.7 for large projects involving many materials, a materials validation plan may be used. Web three (3) options the create a validation master plan. For smaller projects, a materials validation plan is optional. Unlike a written plan for a specific validation project,. Appendices should contain all the relevant documentation referenced or stated in the. Web the validation master plan (vmp) is critical in achieving this goal by documenting compliance requirements and explaining necessary validation activities across a manufacturing operation. You canned creates one terrific protocol, through a template. To see the complete list of the most popular. Want more free medical device resources? Web this template describes the information that needs to be presented in a validation master plan and provides examples. 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). You can create a great protocol, using a template. You canned creates one terrific protocol, through a template. Web a medical device validation master plan (vmp) diagrams the standards associated with the capability of an office, characterizing the areas and systems to be approved, and gives a composed program for achieving and keeping up a certified. To see the complete list of the most popular. • the scope and intended use of the facility • a. Try scribd free for 30 days. With scribd, you can take your ebooks and audibooks anywhere, even offline. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. You can create a great protocol, using a template. Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Web this template describes the information that needs to be presented in a validation master plan and provides examples. 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). This document comprises individual recommendations on four topics relating to equipment qualification and process. Web what is a validation master plan? Learn more & download a free sample here Your pvp should contain the following elements: Web up to 30% cash back the scope of this validation master plan is limited to the following:Validation Master Plan Template Pdf Best of Document Template
Template of a validation plan. Download Scientific Diagram
Master Validation Plan
Validation Master Plan
Validation Master Plan
Validation Master Plan Template Validation Center
Validation Master Plan
Data Validation Plan Template
How to create a Validation Master Plan in 5 steps. Templates & more
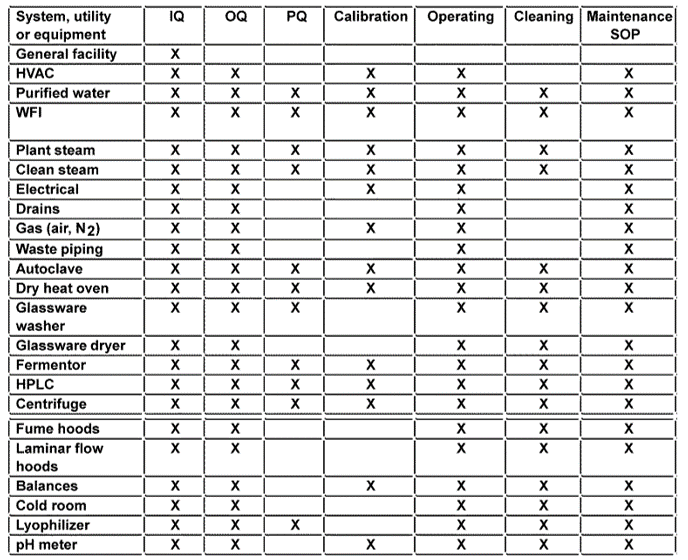
Example of a Validation Master Plan (VMP) Checklist Oriel STAT A
Ad Access Millions Of Ebooks, Audiobooks, Podcasts, And More.
Select To Establish A Validation Master Map Previous The Next Fda Audit?
Web The Basic Principles And Application Of Qualification And Validation Are Described In Annex 15 To The Pic/S And Eu Guide To Gmp.
Current Validation Status For The Systems Within The Project Scope.
Related Post: