Master Manufacturing Record Template
Master Manufacturing Record Template - We have specified them by. Web the master manufacturing record must include: Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. This is the documented and approved set of instructions used to describe how to manufacture a. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. Web suitable for any size manufacturer, this template will enable you to track every detail from the moment you receive an order. Web the master production record is often referred to as the master batch record. Web we have added all the essential documents for agreements and contract to flowcharts related to manufacturing jobs in our template collection. What should a bmr contain? However, it’s so much more than just a place to. To understand how to setup a master production record to understand the details of setting up the manufacturing. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof of each stage.. Master batch records, also known as master production records and master manufacturing records, are version. Web the master manufacturing record must include: To understand how to setup a master production record to understand the details of setting up the manufacturing. Web batch manufacturing records : This is the documented and approved set of instructions used to describe how to manufacture. Ad a modern solution to an old problem. To understand how to setup a master production record to understand the details of setting up the manufacturing. Stop wasting time & money. Web i was just making a template for master manufacturing records template for the company i work for to help out production, and was hoping i could get some. Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even provide. To understand how to setup a master production record to understand the details of setting up the manufacturing. This is the documented and approved set of instructions used to. Web the master manufacturing record must include: Web manufacturing checklist templates and other work aids facilitate process planning, project management, production control, and quality inspection during the manufacturing. Web the information on this page is current as of jan 17, 2023. Web we have added all the essential documents for agreements and contract to flowcharts related to manufacturing jobs in. Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. Web i was just making a template for master manufacturing records template for the company i work for to help out production, and was hoping i could get some more. Web the information on this. To understand how to setup a master production record to understand the details of setting up the manufacturing. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof. Ad a modern solution to an old problem. Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. In the manufacturing industry, master production records may also be referred. Web the master manufacturing record must include: ( a) the name of the dietary supplement to. Web batch manufacturing records : (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web master production records and batch production records have several professional aliases. Web master your master batch records. ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or. Web the master production record is often referred to as the master batch record. To understand how to setup a master production record to understand the details of setting up the manufacturing. Odoo mrp takes your manufacturing operations to the next level. Ad a modern solution to an old problem. ( a) the name of the dietary supplement to be. Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. In the manufacturing industry, master production records may also be referred. ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web the master manufacturing record must include: Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even provide. Web master batch records and batch production records. Web manufacturing checklist templates and other work aids facilitate process planning, project management, production control, and quality inspection during the manufacturing. To understand how to setup a master production record to understand the details of setting up the manufacturing. Web the master production record is often referred to as the master batch record. Web suitable for any size manufacturer, this template will enable you to track every detail from the moment you receive an order. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof of each stage. Not before creative the bmr, chemical and process manufacturers must make another document: This is the documented and approved set of instructions used to describe how to manufacture a. Ad a modern solution to an old problem. Web the master manufacturing record must include: Web the master manufacturing record must include: These are required for each unique. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. What should a bmr contain? Web master batch records and batch production records. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of. Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. Web master production records and batch production records have several professional aliases. This is the documented and approved set of instructions used to describe how to manufacture a. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. Web batch manufacturing records : Web the master manufacturing record must include: Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even provide. Web manufacturing checklist templates and other work aids facilitate process planning, project management, production control, and quality inspection during the manufacturing. Master batch records, also known as master production records and master manufacturing records, are version. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof of each stage. These are required for each unique. Not before creative the bmr, chemical and process manufacturers must make another document:Great Master Production Schedule Excel Spreadsheet Job Application
Mfr
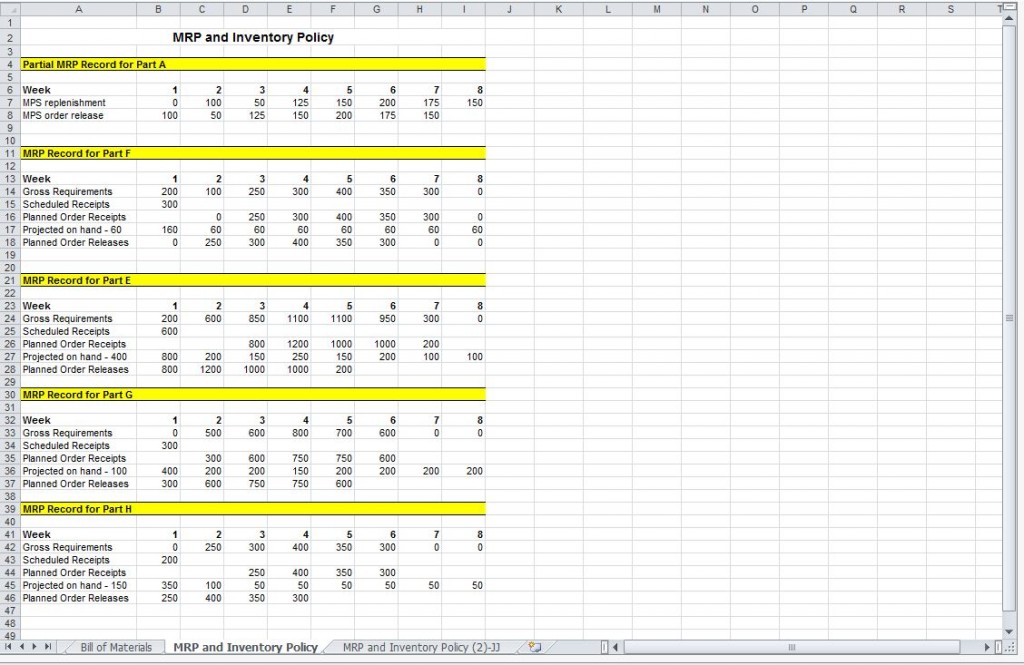
Master Production Schedule Master Production Template
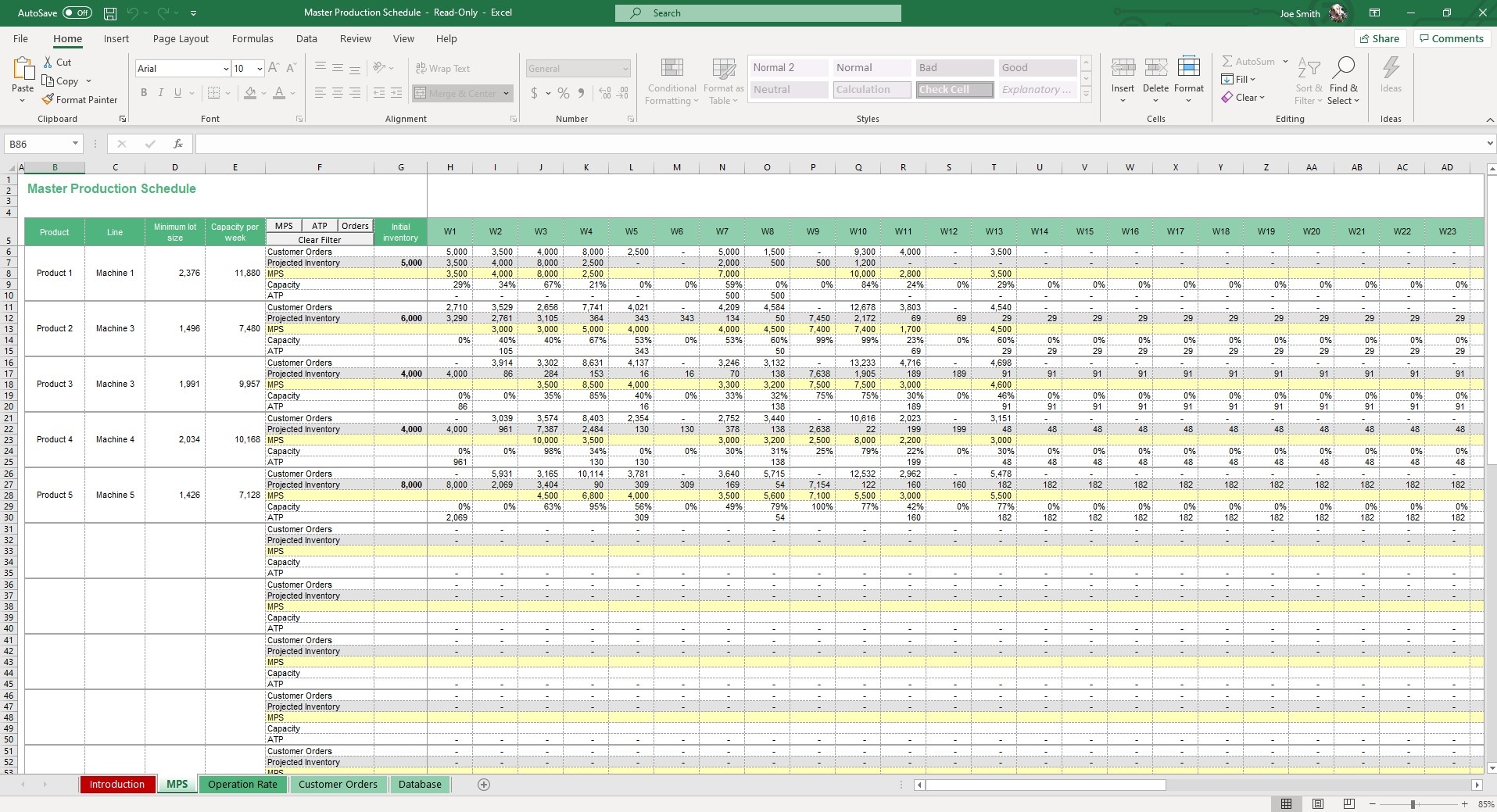
Master Production Schedule (MPS) Excel Template Simple Sheets
Fotos De Doris Villallobos En Evaluación BF8
Device Master Record Procedure
Device Master Record Contents Template
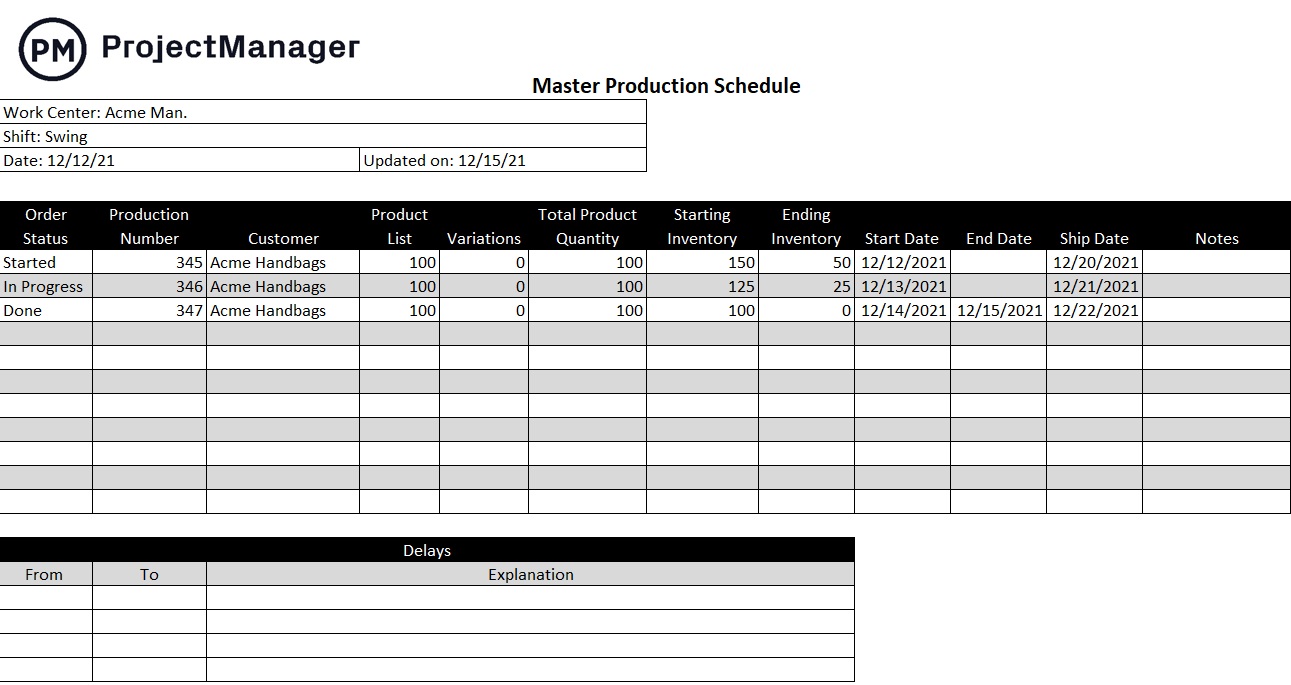
How to Create a Master Production Schedule (MPS) Project Manager News
Master Production Schedule Template Excel Luxury 29 Of Food
PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X
Web The Information On This Page Is Current As Of Jan 17, 2023.
I Am Looking For Templates Of Master Production Record (Per 21 Cfr Part 211.186), And Master Batch Record (Per 21 Cfr Part 211.188).
Web Master Your Master Batch Records.
We Have Specified Them By.
Related Post: