Iso 13485 Management Review Template
Iso 13485 Management Review Template - Document templates contain an average of twenty comments each,. Document templates contain an average of twenty comments each, and offer clear. This sop describes how the surveillance of the quality management system shall be conducted to ensure ongoingadequacy, suitability and efficacy of. Create headings for each of the review inputs from the standard (iso. Web the documentation template may be used for iso 13485 certification audit purposes. Mapping of requirements to documents sven piechottka template download this is a free template, provided by. These templates can include forms for documenting. You don’t need to update your quality manual and objectives a lot. Download our iso 13485 risk management pdf plan template to: Web mark meer said: Web an example iso 13485 risk management plan. Web luckily for you, we have a template built specifically for performing internal audits against the iso management systems, designed in accordance with the. Document templates contain an average of twenty comments each, and offer clear. You don’t need to update your quality manual and objectives a lot. Documents include placeholder marks. Web use this free template when auditing your organization’s management system in accordance with iso 13485. Document templates contain an average of twenty comments each,. A simple formula for a management review report template: Management review report sven piechottka template download this is a free. Web forms and checklists are used to record data, capture information, and facilitate compliance with. Web preview procedure for management review template. These templates can include forms for documenting. Web templates iso 13485 templates updated june 9, 2022 template: A simple formula for a management review report template: Web luckily for you, we have a template built specifically for performing internal audits against the iso management systems, designed in accordance with the. Web luckily for you, we have a template built specifically for performing internal audits against the iso management systems, designed in accordance with the. Documents include placeholder marks for. Web the documentation template may be used for iso 13485 certification audit purposes. Create headings for each of the review inputs from the standard (iso. Web here we propose our management. This sop describes how the surveillance of the quality management system shall be conducted to ensure ongoingadequacy, suitability and efficacy of. Documents include placeholder marks for. Download our iso 13485 risk management pdf plan template to: Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745.. Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745. These templates can include forms for documenting. A simple formula for a management review report template: You don’t need to update your quality manual and objectives a lot. Document templates contain an average of twenty comments. Document templates contain an average of twenty comments each,. Web updated june 9, 2022 template: Web mark meer said: The document is fully editable so that you can adapt it to your company design. Web the documentation template may be used for iso 13485 certification audit purposes. You don’t need to update your quality manual and objectives a lot. Web the documentation template may be used for iso 13485 certification audit purposes. Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745. Web use this free template when auditing your organization’s management system. Management review report sven piechottka template download this is a free. Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745. Web check them out below. Web the documentation template may be used for iso 13485 certification audit purposes. These templates can include forms for documenting. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485 :2016 + a11:2021. Web updated june 9, 2022 template: Web use our free iso 13485 procedure template and the list of iso 13485:2016 mandatory procedures to build your medical device quality system and get certified. These templates can. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485 :2016 + a11:2021. Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Web the advantage of that: Web the documentation template may be used for iso 13485 certification audit purposes. Document templates contain an average of twenty comments each,. This sop describes how the surveillance of the quality management system shall be conducted to ensure ongoingadequacy, suitability and efficacy of. Web use this free template when auditing your organization’s management system in accordance with iso 13485. Web luckily for you, we have a template built specifically for performing internal audits against the iso management systems, designed in accordance with the. Create headings for each of the review inputs from the standard (iso. Download our iso 13485 risk management pdf plan template to: Management review report sven piechottka template download this is a free. Web updated june 9, 2022 template: Web the documentation template may be used for iso 13485 certification audit purposes. Web use our free iso 13485 procedure template and the list of iso 13485:2016 mandatory procedures to build your medical device quality system and get certified. Web preview procedure for management review template. These templates can include forms for documenting. You don’t need to update your quality manual and objectives a lot. Mapping of requirements to documents sven piechottka template download this is a free template, provided by. Documents include placeholder marks for. Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745. Management review report sven piechottka template download this is a free. Document templates contain an average of twenty comments each, and offer clear. Web mark meer said: Document templates contain an average of twenty comments each,. These templates can include forms for documenting. Web preview procedure for management review template. Web the advantage of that: Web the documentation template may be used for iso 13485 certification audit purposes. Web templates iso 13485 templates updated june 9, 2022 template: Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. Web use this free template when auditing your organization’s management system in accordance with iso 13485. Create headings for each of the review inputs from the standard (iso. Web check them out below. You don’t need to update your quality manual and objectives a lot. The document is fully editable so that you can adapt it to your company design. Web here we propose our management review template which is aligned with the requirements of iso 13485, 21 cfr 820 and eu mdr 2017/745.Iso 13485 Quality Manual Template Free
Management Review Procedure
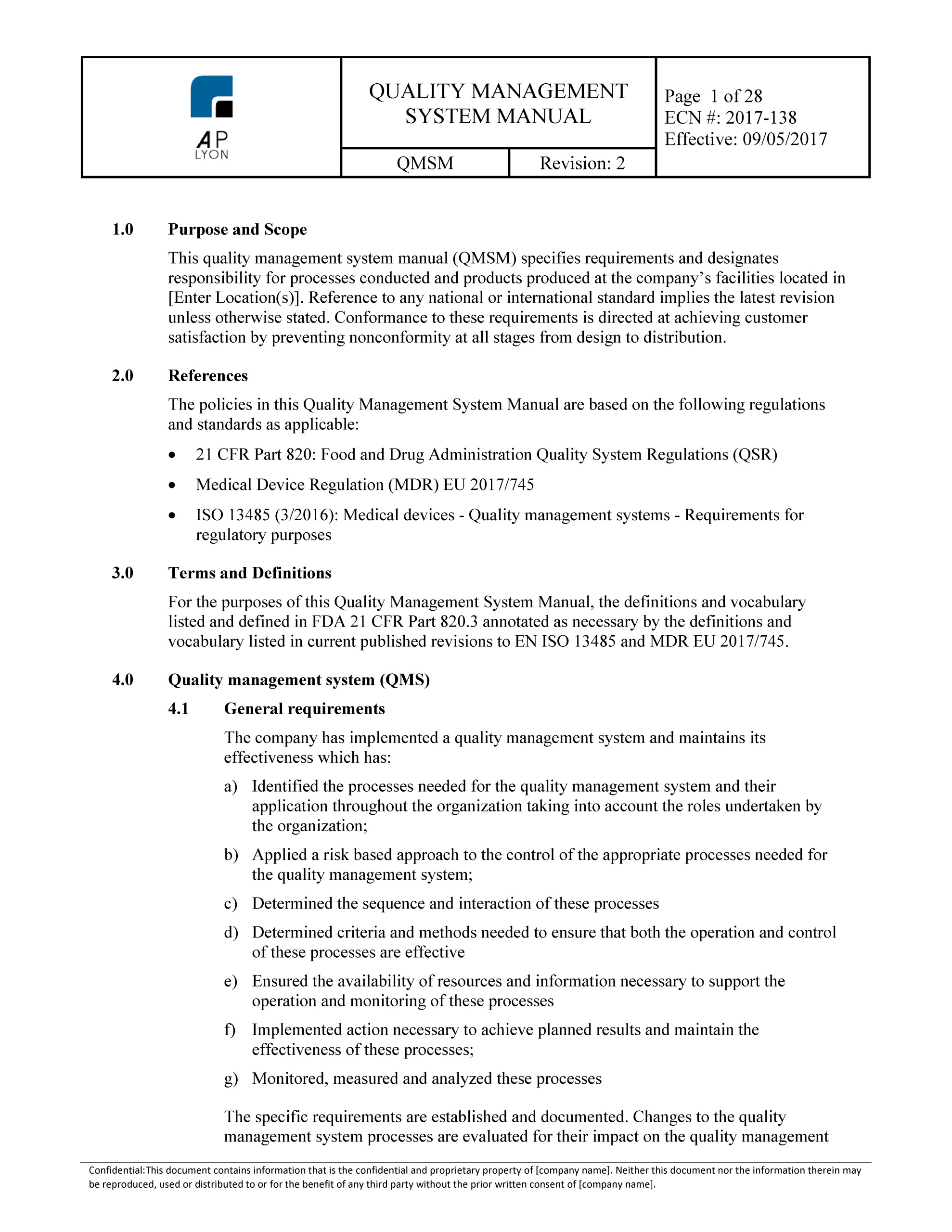
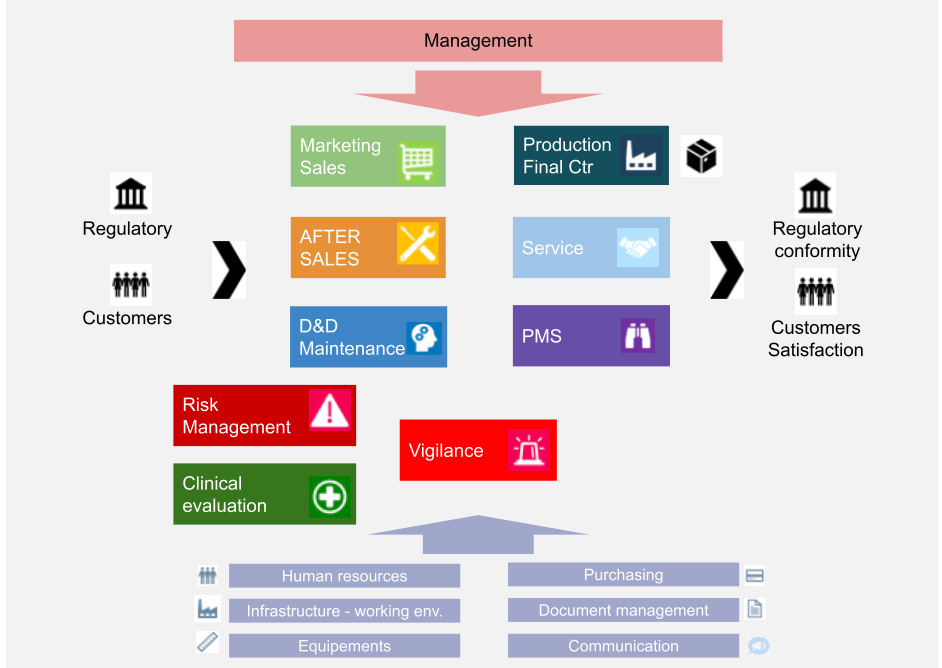
Quality manual, ISO 13485 and MDR, free template
ISO 13485 CERTIFIED IQ Medical ventures BV
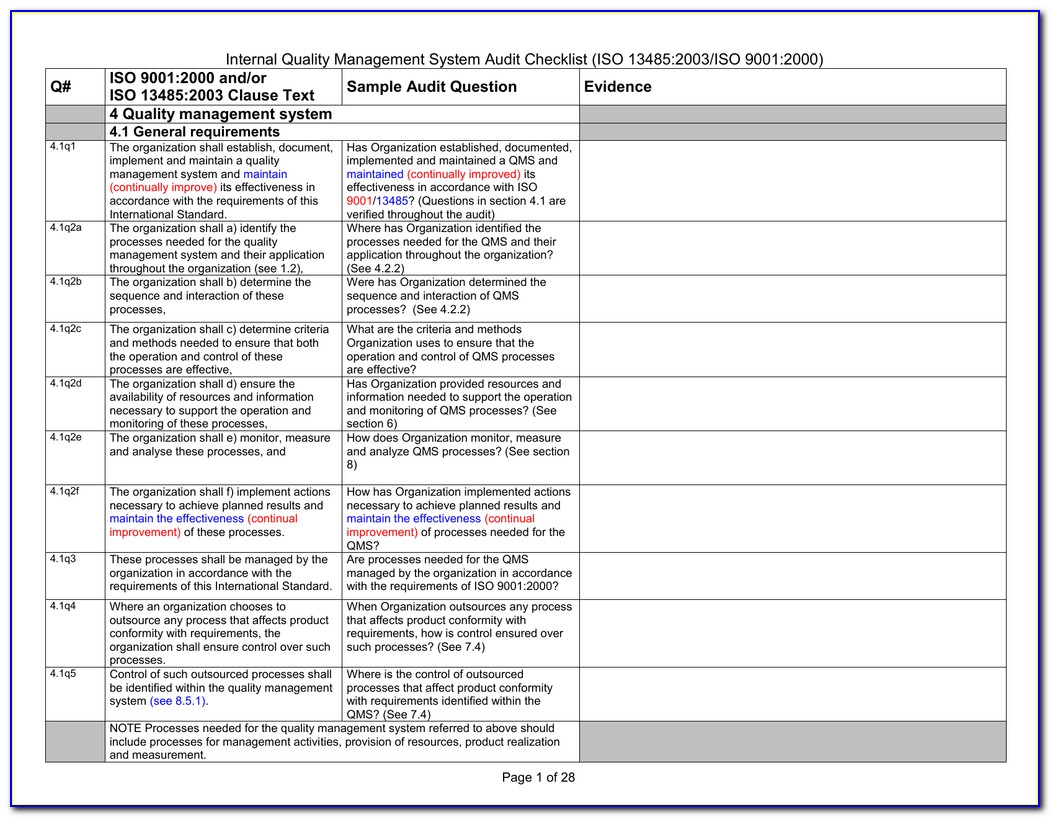
Iso 13485 Audit Report Sample
Iso 13485 Quality Manual Template Free baldcircleao
ISO 13485 Documentation Requirements
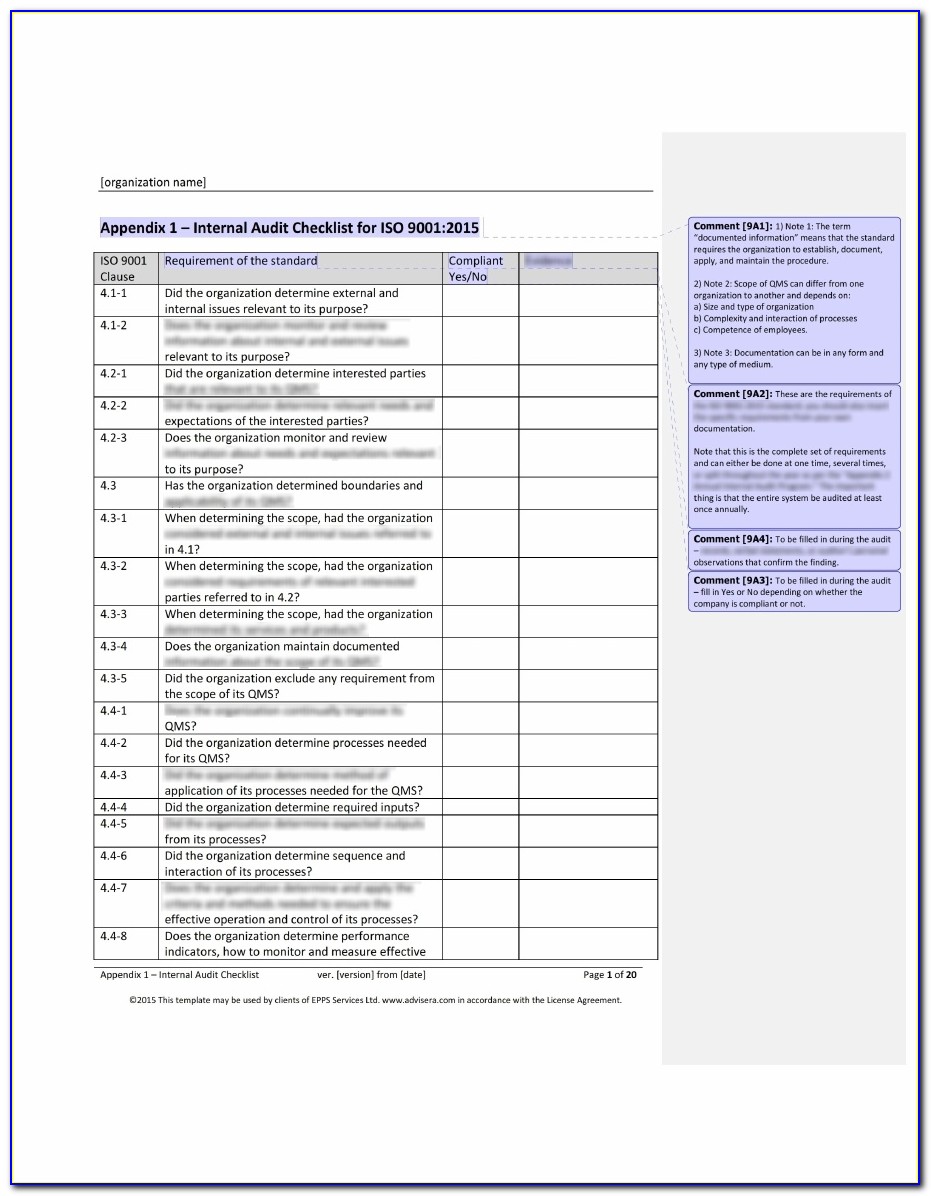
Iso 13485 internal audit checklist hitswes
Iso 13485 internal audit checklist currentvast
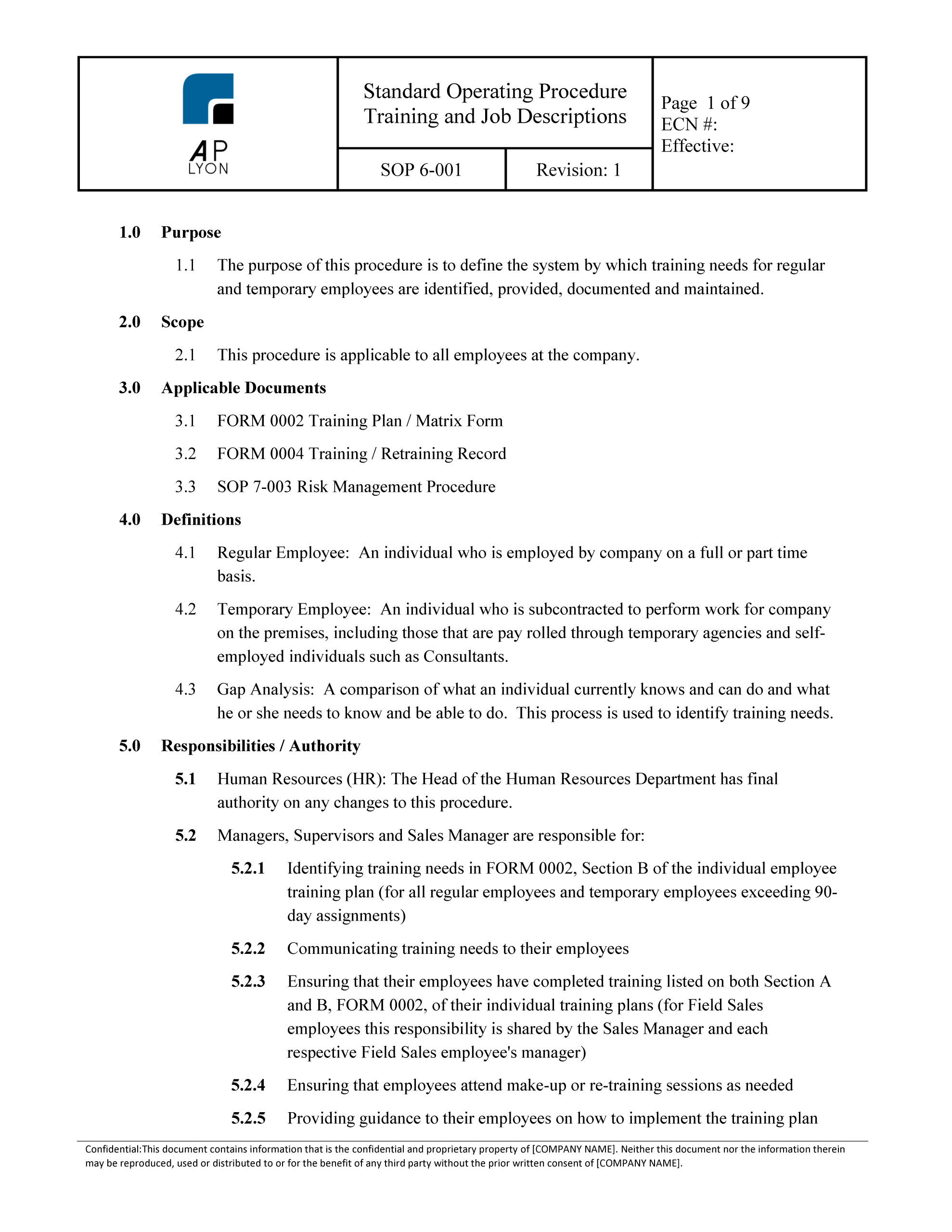
Training Procedure
Web The Documentation Template May Be Used For Iso 13485 Certification Audit Purposes.
Download Our Iso 13485 Risk Management Pdf Plan Template To:
Web Use Our Free Iso 13485 Procedure Template And The List Of Iso 13485:2016 Mandatory Procedures To Build Your Medical Device Quality System And Get Certified.
This Sop Describes How The Surveillance Of The Quality Management System Shall Be Conducted To Ensure Ongoingadequacy, Suitability And Efficacy Of.
Related Post: