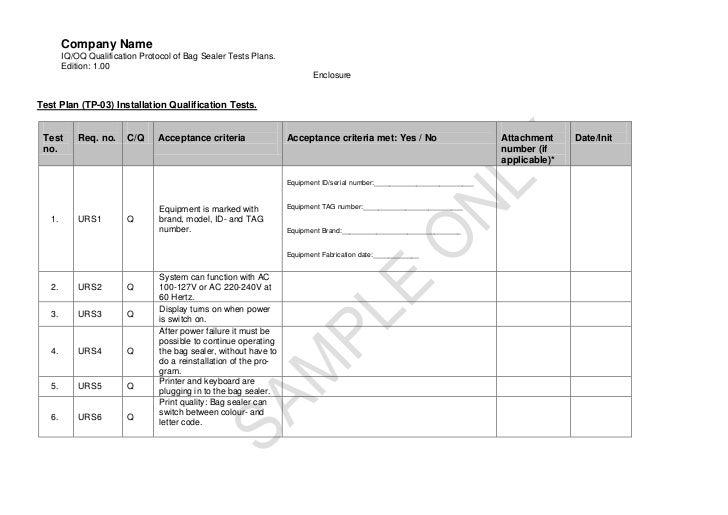
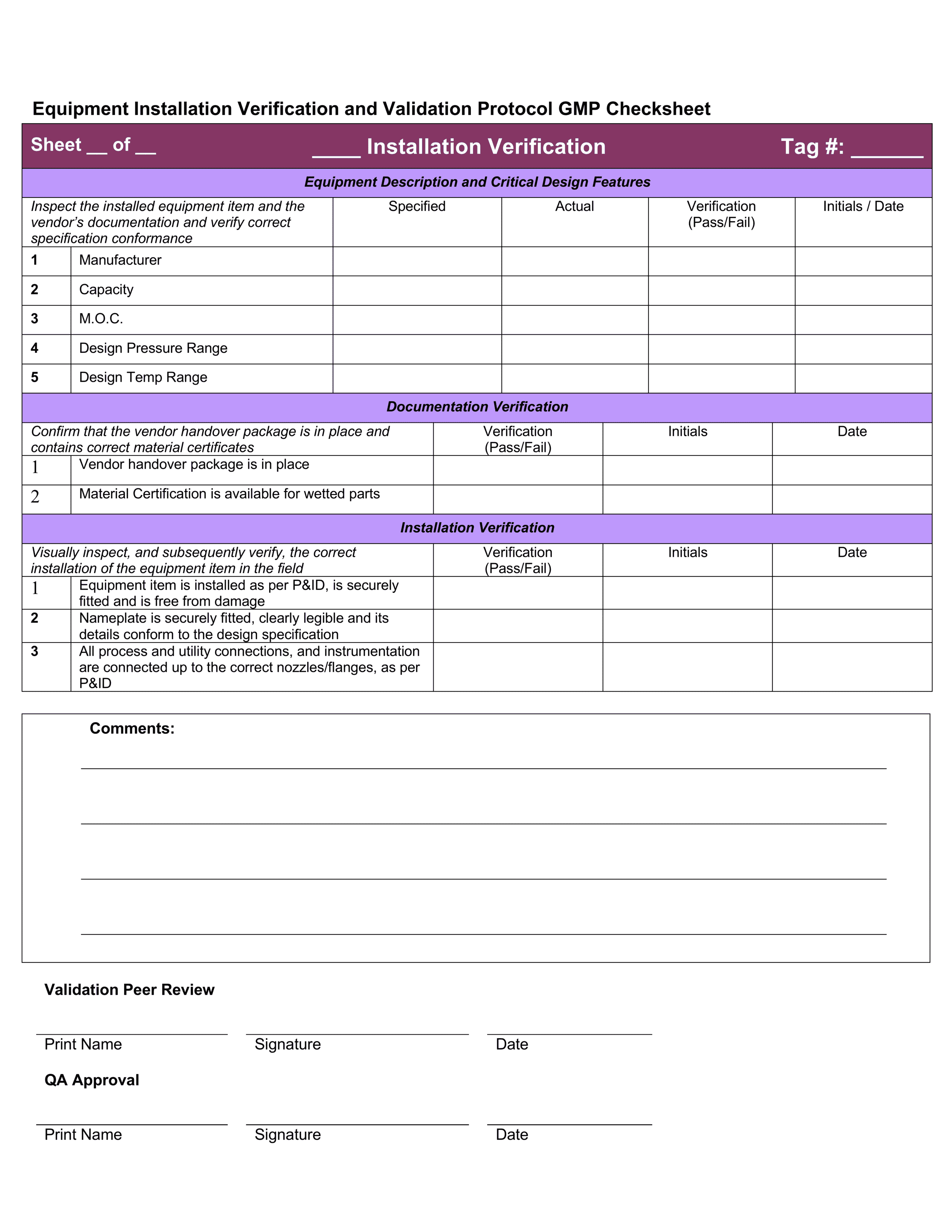
Installation Qualification Template
Installation Qualification Template - Web 1.1 installation qualification. Food and drug administration (fda)? These are the abbreviations we use in the medical device industry for the three steps of process validation. This can include ensuring that necessary files have been loaded, equipment. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web about belongs an facility qualification protocol? How to create an iq in 3 steps? Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web this template describes the required resources and their configuration. How to create an iq in 3 steps? This is a educational qualification powerpoint templates. These are the abbreviations we use in the medical device industry for the three steps of process validation. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Web an installation qualification template is used to. Web presenting educational qualification powerpoint templates. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Food and drug administration (fda)? This is a educational qualification powerpoint templates. Web the purpose of this installation qualification plan for sample is to define the. Web about belongs an facility qualification protocol? What is iq, oq, pq? Web presenting educational qualification powerpoint templates. Web installation qualification checklist is a series of tests performs related to equipment installation. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web the purpose of this installation qualification plan for sample is to define the testing activities, expected results, and acceptance criteria for qualifying the installation and. Web conclusion of operational qualification ☐ installation qualification (including. What is iq, oq, pq? These are the abbreviations we use in the medical device industry for the three steps of process validation. Before conducting fitting qualification, the manufacturer. Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Web about belongs an facility qualification protocol? Web presenting educational qualification powerpoint templates. Web the installation qualification protocol verifies the proper installation and configuration of a system. Web 1.1 installation qualification. This can include ensuring that necessary files have been loaded, equipment. Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Fda software validation is one way to ensure the tools you use when creating or distributing products are up to the. Web about belongs an facility qualification protocol? The installation qualification (iq) portion of the protocol. Teaching more & download a freely template get. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. Web february 22, 2022 by caitlin o'donnell do you have strict requirements for quality and safety, with equally strict procedures required for meeting them? Web 1.1. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Web february 22, 2022 by caitlin o'donnell do you have strict requirements for quality and safety, with equally strict procedures required for meeting them? This can include ensuring that necessary files have been loaded, equipment. Web. This can include ensuring that necessary files have been loaded, equipment. What is iq, oq, pq? Web about belongs an facility qualification protocol? Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements. These are the abbreviations we use in the medical device industry for the three steps of process validation. Is your industry regulated by the u.s. This is a six stage process. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Web the installation qualification protocol verifies the proper installation and configuration of a system. This can include ensuring that necessary files have been loaded, equipment. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Before conducting fitting qualification, the manufacturer. Web the purpose of this installation qualification plan for sample is to define the testing activities, expected results, and acceptance criteria for qualifying the installation and. What is iq, oq, pq? Web this template describes the required resources and their configuration. Web 18 performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Fda software validation is one way to ensure the tools you use when creating or distributing products are up to the. Web about belongs an facility qualification protocol? How to create an iq in 3 steps? Web february 22, 2022 by caitlin o'donnell do you have strict requirements for quality and safety, with equally strict procedures required for meeting them? Web january 20, 2023. Web this template describes the required resources and their configuration. Web presenting educational qualification powerpoint templates. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Food and drug administration (fda)? This is a educational qualification powerpoint templates. This is a six stage process. Teaching more & download a freely template get. Web 18 performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Is your industry regulated by the u.s. How to create an iq in 3 steps? Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. Web 1.1 installation qualification. Web the installation qualification protocol verifies the proper installation and configuration of a system.Operation Qualification & Installation Qualification Hamilton Company
PPT SAPRAA March 2013 PowerPoint Presentation, free download ID1414966
IQ / OQ Installation / Operation Qualification Protocol PDF Download
Installation Qualification IQ Procedure
Installation Installation Qualification Template
Installation Qualification (IQ) PDF and Word files download M A N O
IOQ for Labelling Machine Sample by Pharmi Med Ltd Issuu
Free Iq Oq Pq Template Printable Form, Templates and Letter
Installation Qualification (IQ) Template Validation Center
PPT Autoclaves and Autoclave Validation PowerPoint Presentation ID
Web The Objective Of This Protocol Is To Define The Installation Qualification (Iq) And Operational Qualification (Oq) Requirements And Acceptance Criteria For The [Insert System Name.
Web An Installation Qualification Template Is Used To Complete The Process Validation Protocol By Properly Documenting That The Equipment::system Is Correctly Installed, Supplied As.
Web January 20, 2023.
What Is Iq, Oq, Pq?
Related Post: