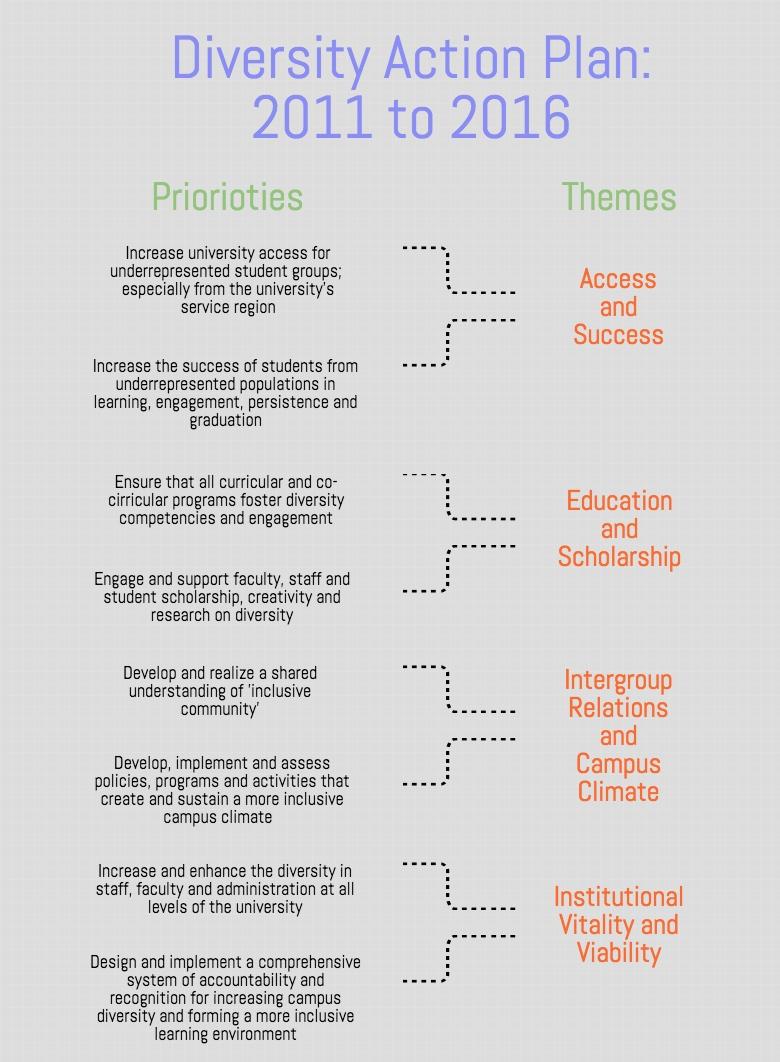
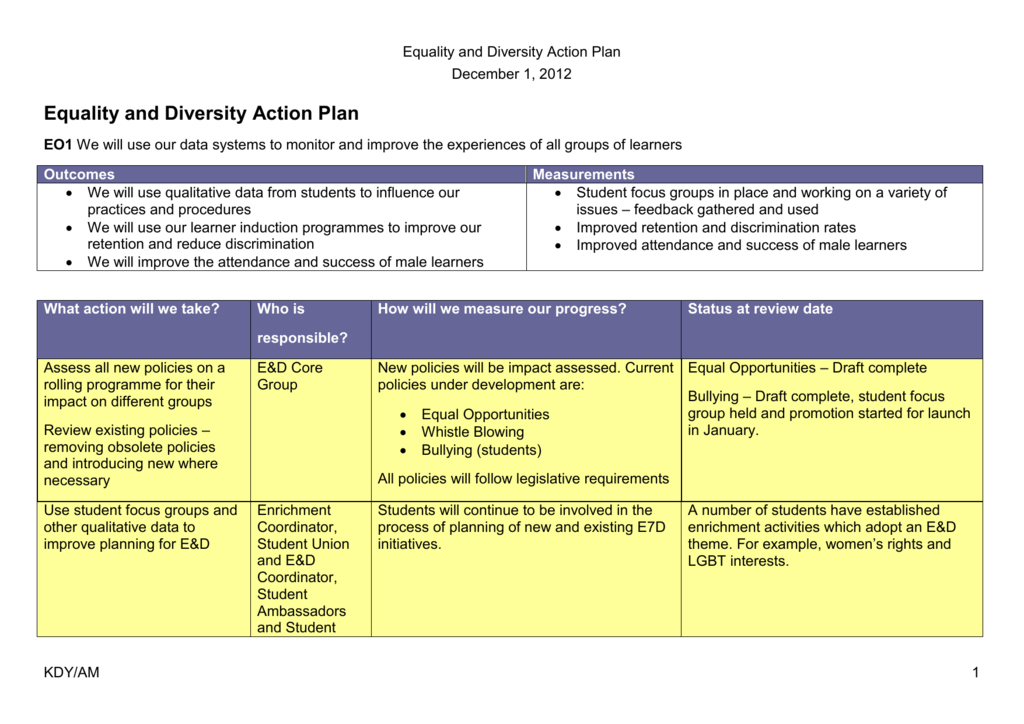
Fda Diversity Plan Template
Fda Diversity Plan Template - Web the combination of the fda's new guidance, recent successes and industry investments, and focus on diversity is creating momentum within the broader trial ecosystem and giving diversity advocates within companies a platform for influencing functional leaders to. Law as part of the 2023 omnibus spending bill, clinical research under american jurisdiction must now comply with the fda diversity plan to achieve racial and ethnic inclusiveness. Katie hobbins | mar 06, 2023. Web diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry draft guidance this guidance document is being. Web april 13, 2022 español today, the u.s. Web office of communication, outreach and development. Food and drug administration issued a new draft guidance to industry for developing plans to enroll more participants from underrepresented racial and. Web the purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing a race and ethnicity diversity plan (referred to as the. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in clinical trials including during the study design phase. Web on april 13, 2022, the food & drug administration (fda) issued a new draft guidance for industry for “developing plans to enroll more participants from underrepresented racial and ethnic populations in the u.s. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in clinical trials including during the study design phase. Web one initiative, the diverse and equitable participation in clinical trials (depict) act would require the fda to revise its regulations to require sponsors of an ind or ide to provide demographic prevalence data, develop enrollment targets,. Law as part of the 2023 omnibus spending bill, clinical research under american jurisdiction must now comply with the fda diversity plan to achieve racial and ethnic inclusiveness. Web blog | may 11, 2022 five things researchers need to know about the fda’s updated diversity plans guidance by praduman pj jain, founder and ceo, vibrent health over the past few. Web fda guidance on enhancing the diversity of clinical trial populations: Web on april 13, 2022, the food & drug administration (fda) issued a new draft guidance for industry for “developing plans to enroll more participants from underrepresented racial and ethnic populations in the u.s. Web the office of digital transformation (odt) diversity, equity, inclusion, and accessibility (deia) action plan. Katie hobbins | mar 06, 2023. Web the purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing a race and ethnicity diversity plan (referred to as the. Web the united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to improve enrollment of participants. Clinical trials are research studies involving human volunteers to evaluate medical products like medications, vaccines, or devices for safety and effectiveness. Web one initiative, the diverse and equitable participation in clinical trials (depict) act would require the fda to revise its regulations to require sponsors of an ind or ide to provide demographic prevalence data, develop enrollment targets, and submit.. Web the purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing a race and ethnicity diversity plan (referred to as the. Web anju team july 11, 2023 with the depict act having been signed into u.s. The food and drug administration (fda) is committed to further developing and establishing an inclusive. 10903 new hampshire ave., bldg. Web clinical trial diversity. Web the food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry entitled “diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials.” In april 2022, fda released a draft guidance on improving enrollment for participants from underrepresented. Web blog | may 11, 2022 five things researchers need to know about the fda’s updated diversity plans guidance by praduman pj jain, founder and ceo, vibrent health over the past few years, the topic of diversity in biomedical research has. Web clinical trial diversity. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in clinical trials including during the study design phase. In april 2022, fda released a draft guidance on improving enrollment for participants from underrepresented racial. Web april 13, 2022 español today, the u.s. Web the purpose of this guidance is to provide recommendations to sponsors developing. Web the food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry entitled “diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials.” Web diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry draft guidance this guidance. Web diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry draft guidance this guidance document is being. Web the office of digital transformation (odt) diversity, equity, inclusion, and accessibility (deia) action plan 2023 is intended to guide the efforts of all odt offices and community members in. Web the united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to improve enrollment of participants from underrepresented. Web the combination of the fda's new guidance, recent successes and industry investments, and focus on diversity is creating momentum within the broader trial ecosystem and giving diversity advocates within companies a platform for influencing functional leaders to. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in clinical trials including during the study design phase. The food and drug administration (fda) is committed to further developing and establishing an inclusive work environment that values human diversity in all its forms. Law as part of the 2023 omnibus spending bill, clinical research under american jurisdiction must now comply with the fda diversity plan to achieve racial and ethnic inclusiveness. 10903 new hampshire ave., bldg. Web on april 13, 2022, the food & drug administration (fda) issued a new draft guidance for industry for “developing plans to enroll more participants from underrepresented racial and ethnic populations in the u.s. Clinical trials are research studies involving human volunteers to evaluate medical products like medications, vaccines, or devices for safety and effectiveness. Web anju team july 11, 2023 with the depict act having been signed into u.s. Web april 13, 2022 español today, the u.s. Web clinical trial diversity. Web fda guidance on enhancing the diversity of clinical trial populations: Food and drug administration issued a new draft guidance to industry for developing plans to enroll more participants from underrepresented racial and. Web office of communication, outreach and development. Center for biologics evaluation and research. Web one initiative, the diverse and equitable participation in clinical trials (depict) act would require the fda to revise its regulations to require sponsors of an ind or ide to provide demographic prevalence data, develop enrollment targets, and submit. Web the purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing a race and ethnicity diversity plan (referred to as the. Web the united states food and drug managing (fda) exposed draft tour upon april 13, 2022, entitled, “diversity floor to improve registry of participants from underrepresented racial and ethnic populations stylish clinical trials” (guidance). Web fda guidance on enhancing the diversity of clinical trial populations: In april 2022, fda released a draft guidance on improving enrollment for participants from underrepresented racial. Law as part of the 2023 omnibus spending bill, clinical research under american jurisdiction must now comply with the fda diversity plan to achieve racial and ethnic inclusiveness. Web blog | may 11, 2022 five things researchers need to know about the fda’s updated diversity plans guidance by praduman pj jain, founder and ceo, vibrent health over the past few years, the topic of diversity in biomedical research has. Eligibility criteria, enrollment practices, and trial designs encourages the inclusion of persons with disabilities in clinical trials including during the study design phase. Clinical trials are research studies involving human volunteers to evaluate medical products like medications, vaccines, or devices for safety and effectiveness. Web anju team july 11, 2023 with the depict act having been signed into u.s. Web the united states food and drug managing (fda) exposed draft tour upon april 13, 2022, entitled, “diversity floor to improve registry of participants from underrepresented racial and ethnic populations stylish clinical trials” (guidance). Web the purpose of this guidance is to provide recommendations to sponsors developing medical products on the approach for developing a race and ethnicity diversity plan (referred to as the. Web the combination of the fda's new guidance, recent successes and industry investments, and focus on diversity is creating momentum within the broader trial ecosystem and giving diversity advocates within companies a platform for influencing functional leaders to. Web clinical trial diversity. Food and drug administration issued a new draft guidance to industry for developing plans to enroll more participants from underrepresented racial and. Web the food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry entitled “diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials.” The food and drug administration (fda) is committed to further developing and establishing an inclusive work environment that values human diversity in all its forms. Web the united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to improve enrollment of participants from underrepresented. Web diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry draft guidance this guidance document is being.The Orion Multiyear diversity plan works, for the most part
Diversity Recruitment Plan Template
Diversity Action Plan Wal Mart
Diversity Action Plan Template Google Docs, Word, Apple Pages
Diversity Recruitment Plan Template
30 Business Plan Presentation Template Hamiltonplastering
Diversity and Inclusion Strategy Scorecard with KPIs in 2021
diversity plan Diversity (Business) Health Care
Diversity Plan Template
UDP Diversity Plan Input Urban Design & Planning
Center For Biologics Evaluation And Research.
Web One Initiative, The Diverse And Equitable Participation In Clinical Trials (Depict) Act Would Require The Fda To Revise Its Regulations To Require Sponsors Of An Ind Or Ide To Provide Demographic Prevalence Data, Develop Enrollment Targets, And Submit.
10903 New Hampshire Ave., Bldg.
Katie Hobbins | Mar 06, 2023.
Related Post: