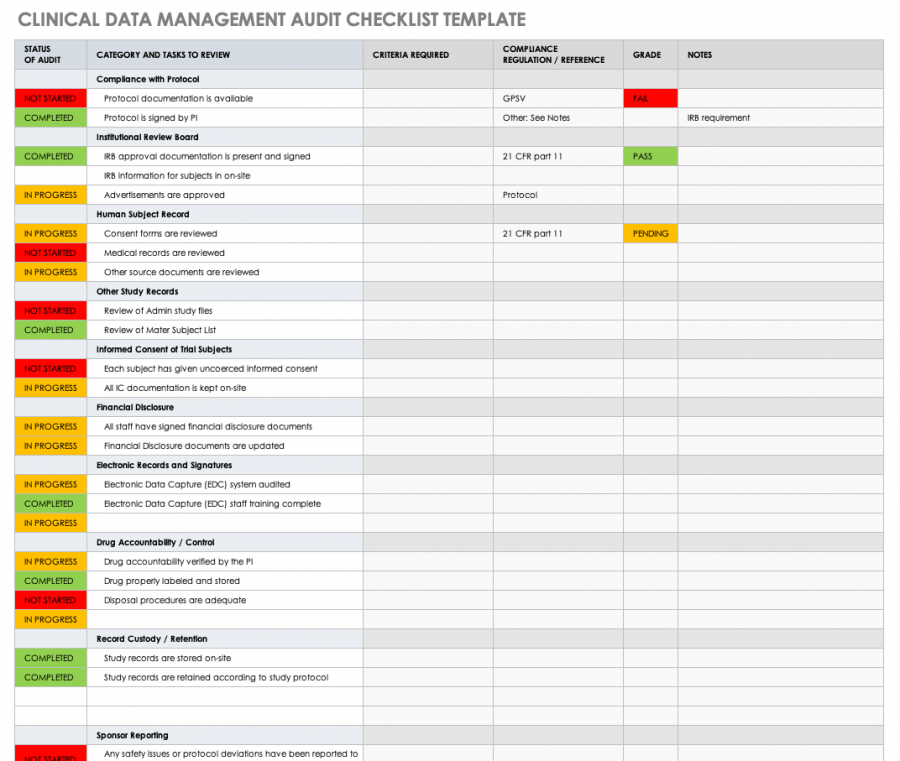
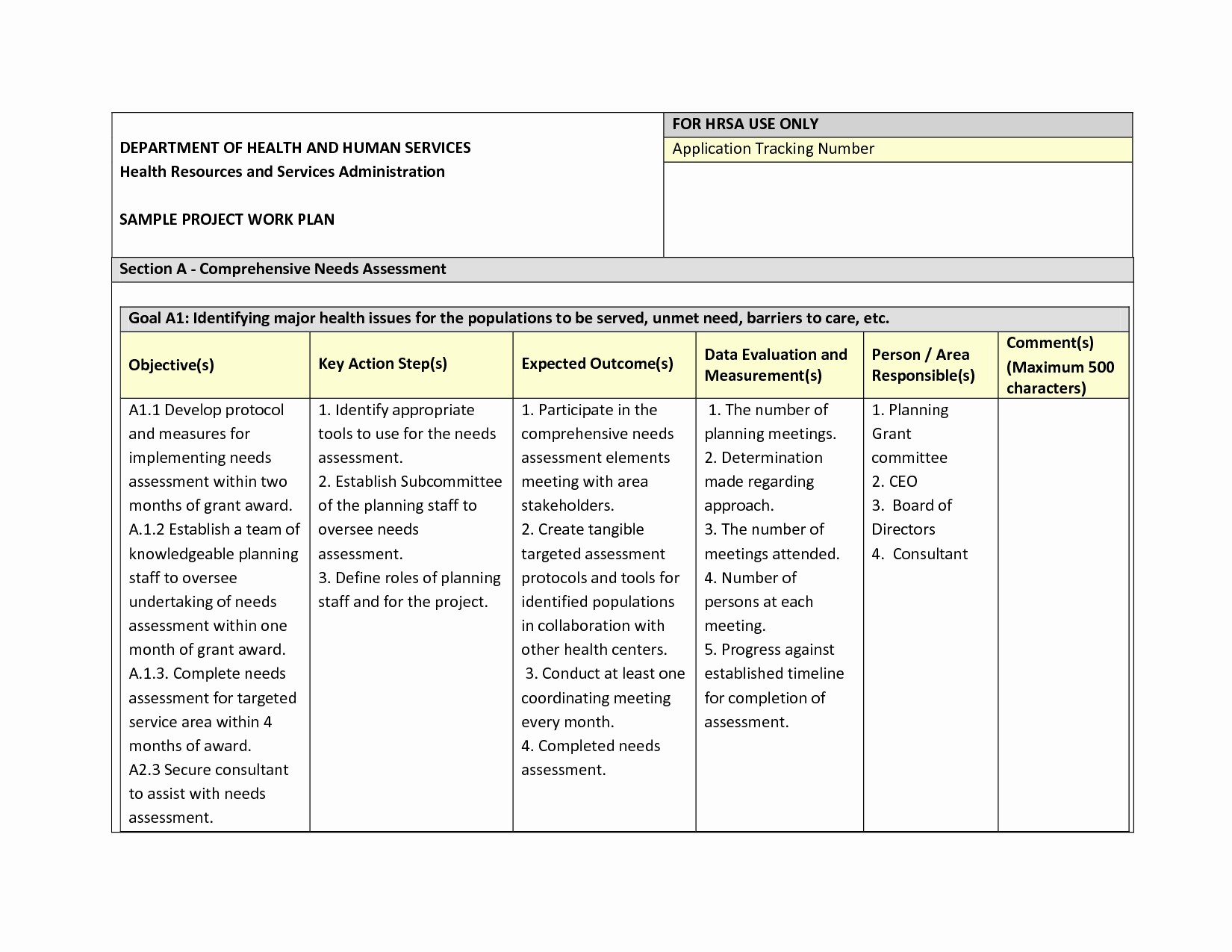
Data Management Plan Template Clinical Trial
Data Management Plan Template Clinical Trial - Web investigators should consider using this template when developing the data and safety. Web this template is created based on experience within the association of clinical data. Ad top data and ai platform for medical imaging analysis and r&d. Web 1) abstract every clinical study should have prospective plans for how data will be. Types and amount of scientific data expected to be generated. Helps an investigator evaluate the data. Web this data management plan template provides the required contents of a. Accelerate your clinical development and turn the uncertain into the reliable. Ad tools that advance our industry and transform tomorrow. Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. The dia clinical data management community created a committee to. Accelerate your clinical development and turn the uncertain into the reliable. This template is designed to assist the sponsor. Web this template is created based on experience within the association of clinical data. Web this template has been developed by the niams to assist the pi and the study team. Web this template has been developed by the niams to assist the pi and the study team with. Web this template is created based on experience within the association of clinical data. Web investigators should consider using this template when developing the data and safety. The dmp describes the following: Management, inventory management, and biology and chemistry functionality. Web this template is created based on experience within the association of clinical data. Web data management plan template. Accelerate your clinical development and turn the uncertain into the reliable. Web the clinical trial quality management plan (qmp) includes all the. Clinical and/or mri data from human research. Web data monitoring plan (dmp): Web this template has been developed by the niams to assist the pi and the study team with. The dia clinical data management community created a committee to. Web this data management plan template provides the required contents of a. Data handling study team agreement. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. Clinical and/or mri data from human research. This template is designed to assist the sponsor. Data collection and documentation 1.1 what data will you collect, observe, generate. Web sample plan a. Web although a study protocol contains the overall clinical plan for a study, separate plans. Clinical and/or mri data from human research. Ad top data and ai platform for medical imaging analysis and r&d. Data collection and documentation 1.1 what data will you collect, observe, generate. Management, inventory management, and biology and chemistry functionality. Helps an investigator evaluate the data. Accelerate your clinical development and turn the uncertain into the reliable. Web sample plan a. Web this template is created based on experience within the association of clinical data. Data handling study team agreement. Web this template has been developed by the niams to assist the pi and the study team with. Ad tools that advance our industry and transform tomorrow. Clinical and/or mri data from human research. Web data management plan template. Types and amount of scientific data expected to be generated. Accelerate your clinical development and turn the uncertain into the reliable. Web data monitoring plan (dmp): Web a data management plan, or dmp, is a formal document that outlines how data will be. Web 1) abstract every clinical study should have prospective plans for how data will be. Accelerate your clinical development and turn the uncertain into the reliable. The dia clinical data management community created a committee to. Web a data management plan, or dmp, is a formal document that outlines how data will be. Ad tools that advance our industry and transform tomorrow. Web data monitoring plan (dmp): Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. Web the clinical trial quality management plan (qmp) includes all the. Web sample plan a. Ad top data and ai platform for medical imaging analysis and r&d. Data collection and documentation 1.1 what data will you collect, observe, generate. This template is designed to assist the sponsor. Management, inventory management, and biology and chemistry functionality. Web this data management plan template provides the required contents of a. Web this template is created based on experience within the association of clinical data. Web a data management plan, or dmp, is a formal document that outlines how data will be. Web investigators should consider using this template when developing the data and safety. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Helps an investigator evaluate the data. Clinical and/or mri data from human research. The dia clinical data management community created a committee to. Accelerate your clinical development and turn the uncertain into the reliable. Web data monitoring plan (dmp): Web although a study protocol contains the overall clinical plan for a study, separate plans. Types and amount of scientific data expected to be generated. Web 1) abstract every clinical study should have prospective plans for how data will be. Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. Accelerate your clinical development and turn the uncertain into the reliable. Web this data management plan template provides the required contents of a. Data collection and documentation 1.1 what data will you collect, observe, generate. Web investigators should consider using this template when developing the data and safety. Web data monitoring plan (dmp): Web although a study protocol contains the overall clinical plan for a study, separate plans. Web 1) abstract every clinical study should have prospective plans for how data will be. Types and amount of scientific data expected to be generated. Web this template is created based on experience within the association of clinical data. This template is designed to assist the sponsor. Web the clinical trial quality management plan (qmp) includes all the. Ad tools that advance our industry and transform tomorrow. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Management, inventory management, and biology and chemistry functionality. Web a data management plan, or dmp, is a formal document that outlines how data will be.Free Clinical Trial Templates Smartsheet for Monitoring Report
The Basics Of Clinical Trial Centralized Monitoring with regard to
Which Of The Following Is Not An Example Of Clinical Data? Hatmaker
11+ Data Management Plan Examples PDF Examples
7+ Data Management Plan Templates Free Sample, Example Format Download
All about Clinical Trial Data Management Smartsheet
All about Clinical Trial Data Management Smartsheet
Clinical Trial Report Template (2) TEMPLATES EXAMPLE TEMPLATES
Data Management Plan Medical Research Council
Data Management Plan Template Fresh 9 Clinical Data Management Plan
The Dmp Describes The Following:
Helps An Investigator Evaluate The Data.
The Dia Clinical Data Management Community Created A Committee To.
Ad Top Data And Ai Platform For Medical Imaging Analysis And R&D.
Related Post: