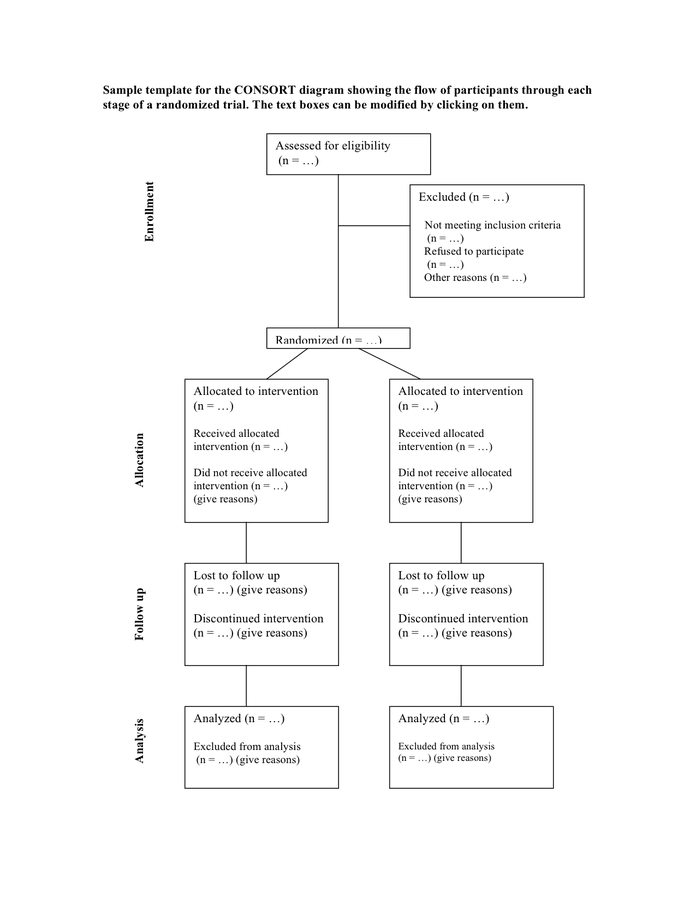
Consort Flow Diagram Template
Consort Flow Diagram Template - 74.2 kb | 50.9 kb Information required to complete a consort flow diagram includes the. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web edit consort diagram template ppt. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. The text boxes can be modified by clicking on them. Web sample template for the consort diagram filetype: Many leading medical journals and major international editorial. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. The consort 2010 checklist is. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Information required to complete a consort flow diagram includes the.. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Information required to complete a consort flow diagram includes the. Web edit consort diagram template ppt. Web sample template for the consort diagram filetype: Web the template for the consort flow diagram [1,2] is. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. Manuscripts reporting the. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). The text boxes can be modified by clicking on them. Web sample template for the. The text boxes can be modified by clicking on them. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. Web consort flow diagram and checklist: Web the statement. Many leading medical journals and major international editorial. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Web consort 2010 flow diagram consort 2010 flow diagram. Web sample template for the consort diagram filetype: Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. Many leading medical journals and major international editorial. Web consort diagram presents the flow of subjects at each stage in a. It offers a standard way for authors to prepare reports of trial. Information required to complete a consort flow diagram includes the. Web consort flow diagram and checklist: Web the template for the consort flow diagram [1,2] is shown in figure figure1. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the. Web sample template for the consort diagram filetype: Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Many leading medical journals and major international editorial. Manuscripts reporting the results of randomized trials must. Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did.. 74.2 kb | 50.9 kb Information required to complete a consort flow diagram includes the. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web consort diagram presents the flow of subjects at each stage in a clinical trial. Web edit consort diagram template ppt. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. Web consort flow diagram and checklist: Manuscripts reporting the results of randomized trials must include the consort flow diagram showing the progress of patients. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. Quickly add and underline text, insert pictures, checkmarks, and signs, drop new fillable areas, and rearrange or delete pages from your. Web sample template for the consort diagram filetype: Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). The text boxes can be modified by clicking on them. The consort 2010 checklist is. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web we present the consort extension to randomised crossover trials, which aims to facilitate better reporting of crossover trials. Web the consort flow diagrams are often included in the clinical study report to provide a bird’s eye view of the flow of patients through the different stages of the trials. 74.2 kb | 50.9 kb Web a consort diagram shows the flow of subjects through each stage in a clinical trial. Web sample template for the consort diagram filetype: Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. Information required to complete a consort flow diagram includes the. Web consort flow diagram and checklist: It offers a standard way for authors to prepare reports of trial. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. The text boxes can be modified by clicking on them. Web the flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical journals. Descriptive and frequency statistics were calculated for both the trial characteristics and for the reporting adherence against the consort flow. Web consort 2010 flow diagram consort 2010 flow diagram enrollment assessed for eligibility (n= ) allocated to intervention (n= ) received allocated intervention (n= ) did. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009).CONSORT Participant Flow Diagram. Download Scientific Diagram
Revised template of the CONSORT diagram showing the flow of
CONSORT 2010 flow diagram. Download Scientific Diagram
Consort flow diagram of the progress through the phases of a 2group
Sample template for the consort diagram in Word and Pdf formats
CONSORT flow diagram 16 . Download Scientific Diagram
CONSORT flow diagram. Download Scientific Diagram
CONSORT 2010 flow diagram. CONSORT flow diagram template courtesy of
CONSORT diagram showing the flow of participants through each stage of
The CONSORT flow diagram depicts the flow of patients through a
Many Leading Medical Journals And Major International Editorial.
Web Edit Consort Diagram Template Ppt.
Web Consort Diagram Presents The Flow Of Subjects At Each Stage In A Clinical Trial.
Quickly Add And Underline Text, Insert Pictures, Checkmarks, And Signs, Drop New Fillable Areas, And Rearrange Or Delete Pages From Your.
Related Post: