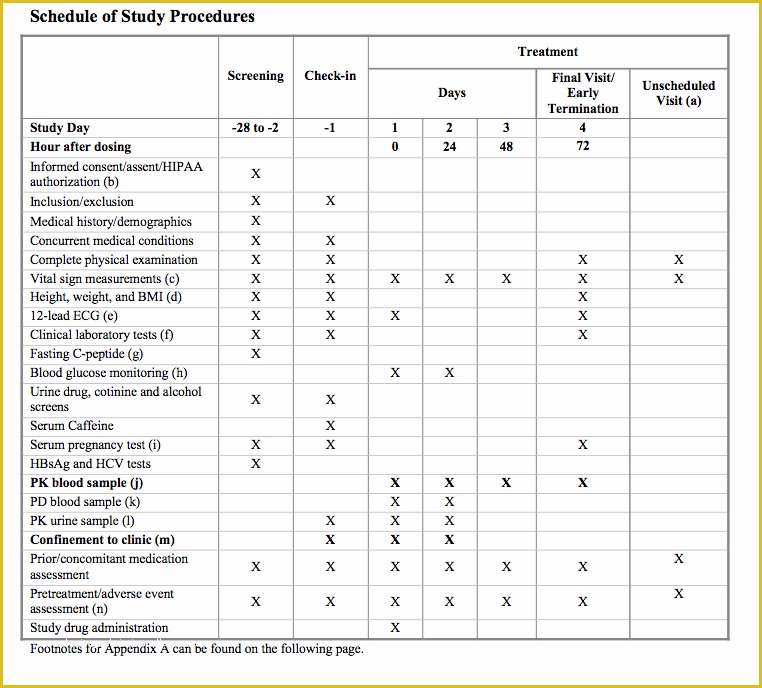
Clinical Study Protocol Template
Clinical Study Protocol Template - Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Phase 2 or 3 clinical trials. Web the clinical intervention study protocol template is a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use. Web a study protocol is an important document that specifies the research plan for a clinical study. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol (version 00) protocol no. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Simplepractice ehr for therapists now equipped with wiley treatment planners. Web use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. Web sample protocol templates and resources: Web use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. Protocol template for behavioral & science research [377kb word file] optional. Web sample protocol templates and resources: Web purpose of the study protocol. Simplepractice ehr for therapists now equipped with wiley treatment planners. Simplepractice ehr for therapists now equipped with wiley treatment planners. Web purpose of the study protocol. Phase 2 or 3 clinical trials. Web sample protocol templates and resources: By following the guidance set out in our structured study protocol template. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: By following the guidance set out in our structured study protocol template. National center for complementary and. Many funders such as the nhs health research authority. It contains sample text to. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Ad streamline treatment plan documentation & improve your patient's progress & experience. Simplepractice ehr for therapists now equipped with wiley treatment planners. Web study protocols reporting a clinical trial can be formatted for submission to trials in two ways: Many funders. Format for clinical trials sponsored by the. Web specifically focused on the translation of laboratory and/or clinical research into new interventions that improve clinical outcomes (e.g., new diagnostics or. Phase 2 or 3 clinical trials. Ad streamline treatment plan documentation & improve your patient's progress & experience. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol (version 00) protocol no. Web sample protocol templates and resources: By following the guidance set out in our structured study protocol template. Ad streamline treatment plan documentation & improve your patient's progress & experience. Center for drug evaluation and research, office of regulatory. Protocol template for behavioral & science research [377kb word file] optional. Web the core protocol is designed as a template for phase 2/3 clinical trials in vwm with the purpose to collect safety, tolerability, and efficacy data for marketing. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and. National center for complementary and. It contains sample text to. Web the clinical intervention study protocol template is a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use. Web sample protocol templates and resources: Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol. Phase 2 or 3 clinical trials. Web the core protocol is designed as a template for phase 2/3 clinical trials in vwm with the purpose to collect safety, tolerability, and efficacy data for marketing. Web specifically focused on the translation of laboratory and/or clinical research into new interventions that improve clinical outcomes (e.g., new diagnostics or. Web the clinical intervention. Many funders such as the nhs health research authority. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Web use this free clinical trial agreement template to. Many funders such as the nhs health research authority. It contains sample text to. Simplepractice ehr for therapists now equipped with wiley treatment planners. Web study protocols reporting a clinical trial can be formatted for submission to trials in two ways: Protocol template for behavioral & science research [377kb word file] optional. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol (version 00) protocol no. Phase 2 or 3 clinical trials. Format for clinical trials sponsored by the. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web the core protocol is designed as a template for phase 2/3 clinical trials in vwm with the purpose to collect safety, tolerability, and efficacy data for marketing. Web purpose of the study protocol. By following the guidance set out in our structured study protocol template. Web a study protocol is an important document that specifies the research plan for a clinical study. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Web the clinical intervention study protocol template is a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use. Web this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Ad streamline treatment plan documentation & improve your patient's progress & experience. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Upon the occurrence of an event qualifying. Web the clinical intervention study protocol template is a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Many funders such as the nhs health research authority. It contains sample text to. Ad streamline treatment plan documentation & improve your patient's progress & experience. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web a study protocol is an important document that specifies the research plan for a clinical study. Web the core protocol is designed as a template for phase 2/3 clinical trials in vwm with the purpose to collect safety, tolerability, and efficacy data for marketing. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. Web clinical trial protocol template version 3.2 ( july 2016) novartis confidential page 2 clinical trial protocol (version 00) protocol no. Simplepractice ehr for therapists now equipped with wiley treatment planners. By following the guidance set out in our structured study protocol template. Phase 2 or 3 clinical trials. National center for complementary and. Protocol template for behavioral & science research [377kb word file] optional. Format for clinical trials sponsored by the.(PDF) Development and Implementation of Clinical Trial Protocol
Clinical Trial Protocol
Phylotocol template. Based on the NIH clinical trial protocol
Clinical Research sop Template Free Of Ich Gcp E6 R2 Addendum Risk
Clinical Trial Protocol Template Ema
Clinical Interventional Study Protocol Template Doc Template pdfFiller
Clinical Trial Protocol
Ich Format for a Clinical Trial Protocol Clinical Trial Statistics
Study Protocol Template Gambaran
KHP CTU Protocol Template
Web Specifically Focused On The Translation Of Laboratory And/Or Clinical Research Into New Interventions That Improve Clinical Outcomes (E.g., New Diagnostics Or.
Web The Ich M11 Clinical Electronic Structured Harmonised Protocol Template Provides Comprehensive Clinical Protocol Organization With Standardized Content With.
Web Study Protocols Reporting A Clinical Trial Can Be Formatted For Submission To Trials In Two Ways:
Upon The Occurrence Of An Event Qualifying.
Related Post: