Capa Report Template
Capa Report Template - Ad free shipping on qualified orders. 4) mobile app to help by. Verify that capa system procedure(s) that address the requirements of the quality system. Access and use your report form on laptop, mobile or tablet. Web prevent recurrence and occurrence of product failures and workplace risks with these capa templates. 2) risks management plan template; Find deals and low prices on capa study guide at amazon.com A capa report is flexible and can be used for different types of issues and incidents. Web a capa report provides a consistent vehicle for recording defects and issues as well as the method of their correction. A capa form records the occurrence. 2) risks management plan template; Web corrective and preventive actions (capa) inspectional objectives. Instantly format your completed corrective and. A capa form records the occurrence. However, not every event warrants a capa report. Instantly format your completed corrective and. A capa report is flexible and can be used for different types of issues and incidents. Web guide to capa reports. However, not every event warrants a capa report. Web 3 of the most capacity account templates: Verify that capa system procedure(s) that address the requirements of the quality system. If you want to complete your own capa reports, here are some steps you can take: Web you can get started writing your own report using our capa report template. Web save time with this customizable template that will help you understand the necessary information and tasks. Access and use your report form on laptop, mobile or tablet. A very important tool during the capa process is the capa form, especially in highly regulated life science industries. However, not every event warrants a capa report. A capa report is flexible and can be used for different types of issues and incidents. Formslaw.com has been visited by 10k+. Find deals and low prices on capa study guide at amazon.com Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Access and use your report form on laptop, mobile or tablet. Usual details include where the problem. Web you can get started writing your own report using our capa report. Ad free shipping on qualified orders. 2) risks management plan template; The first step to completing. Web you can get started writing your own report using our capa report template. Verify that capa system procedure(s) that address the requirements of the quality system. Ad free shipping on qualified orders. Web guide to capa reports. Various events may lead to creation of capa. 4) mobile app to help by. This free capa workflow report. Quality teams must utilize risk managementtechniques to determine the severity of an incident and decide if a capa report is needed. Usual details include where the problem. Web prevent recurrence and occurrence of product failures and workplace risks with these capa templates. As already noted above, there are seven steps to writing an effective capa investigation: Web create effective capa. Web guide to capa reports. 2) risks management plan template; Web 3 of the most capacity account templates: A capa form records the occurrence. The report must begin with a statement of the. 4) mobile app to help by. Find deals and low prices on capa study guide at amazon.com A very important tool during the capa process is the capa form, especially in highly regulated life science industries. As already noted above, there are seven steps to writing an effective capa investigation: Instantly format your completed corrective and. Ad free shipping on qualified orders. Instantly format your completed corrective and. If you want to complete your own capa reports, here are some steps you can take: Verify that capa system procedure(s) that address the requirements of the quality system. The first step to completing. Web corrective and preventive actions (capa) inspectional objectives. Find deals and low prices on capa study guide at amazon.com Various events may lead to creation of capa. As already noted above, there are seven steps to writing an effective capa investigation: Web create effective capa forms using a simple template. Access and use your report form on laptop, mobile or tablet. Web save time with this customizable template that will help you understand the necessary information and tasks to streamline your capa process. The report must begin with a statement of the. Formslaw.com has been visited by 10k+ users in the past month Quality teams must utilize risk managementtechniques to determine the severity of an incident and decide if a capa report is needed. Web you can get started writing your own report using our capa report template. Web a capa report provides a consistent vehicle for recording defects and issues as well as the method of their correction. 2) risks management plan template; A capa report is flexible and can be used for different types of issues and incidents. This free capa workflow report. As already noted above, there are seven steps to writing an effective capa investigation: This free capa workflow report. Web save time with this customizable template that will help you understand the necessary information and tasks to streamline your capa process. Web you can get started writing your own report using our capa report template. Web prevent recurrence and occurrence of product failures and workplace risks with these capa templates. Web a capa report provides a consistent vehicle for recording defects and issues as well as the method of their correction. A capa form records the occurrence. However, not every event warrants a capa report. Access and use your report form on laptop, mobile or tablet. Web create effective capa forms using a simple template. A capa report is flexible and can be used for different types of issues and incidents. 2) risks management plan template; Web 3 of the most capacity account templates: The first step to completing. Instantly format your completed corrective and. Formslaw.com has been visited by 10k+ users in the past monthFree Corrective Action Plan Template Awesome 8 Corrective Action Report
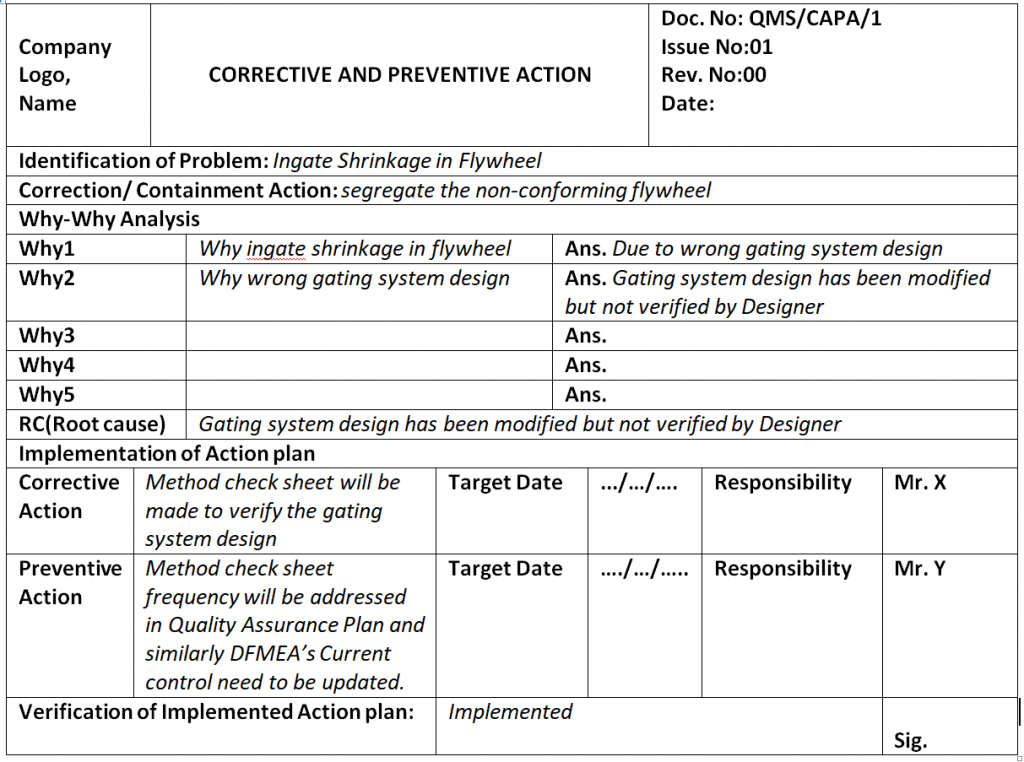
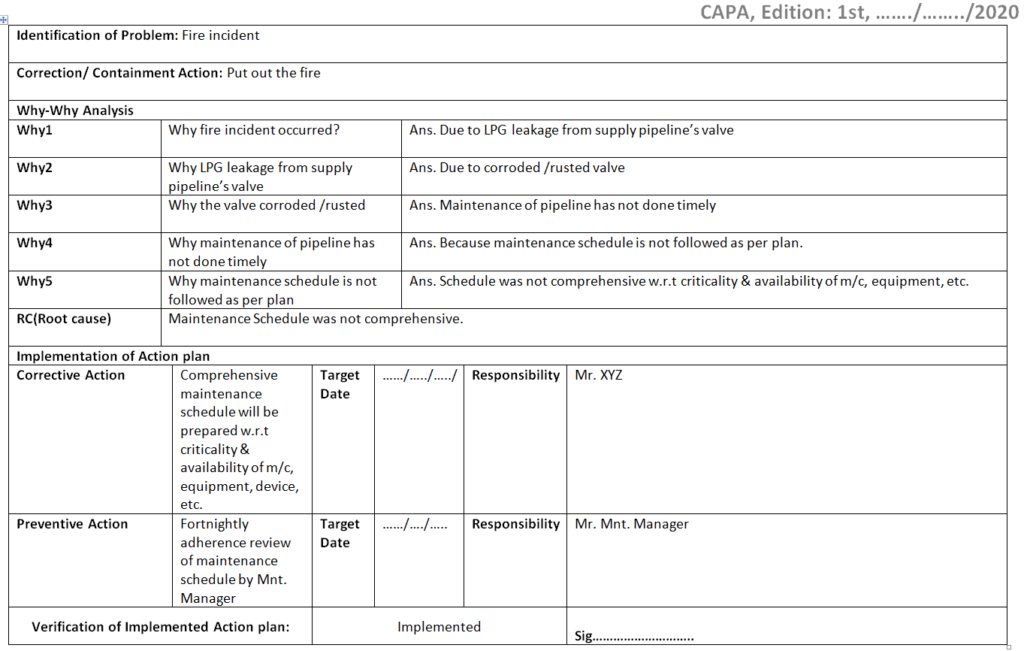
Corrective and Preventive Action Format CAPA with Example
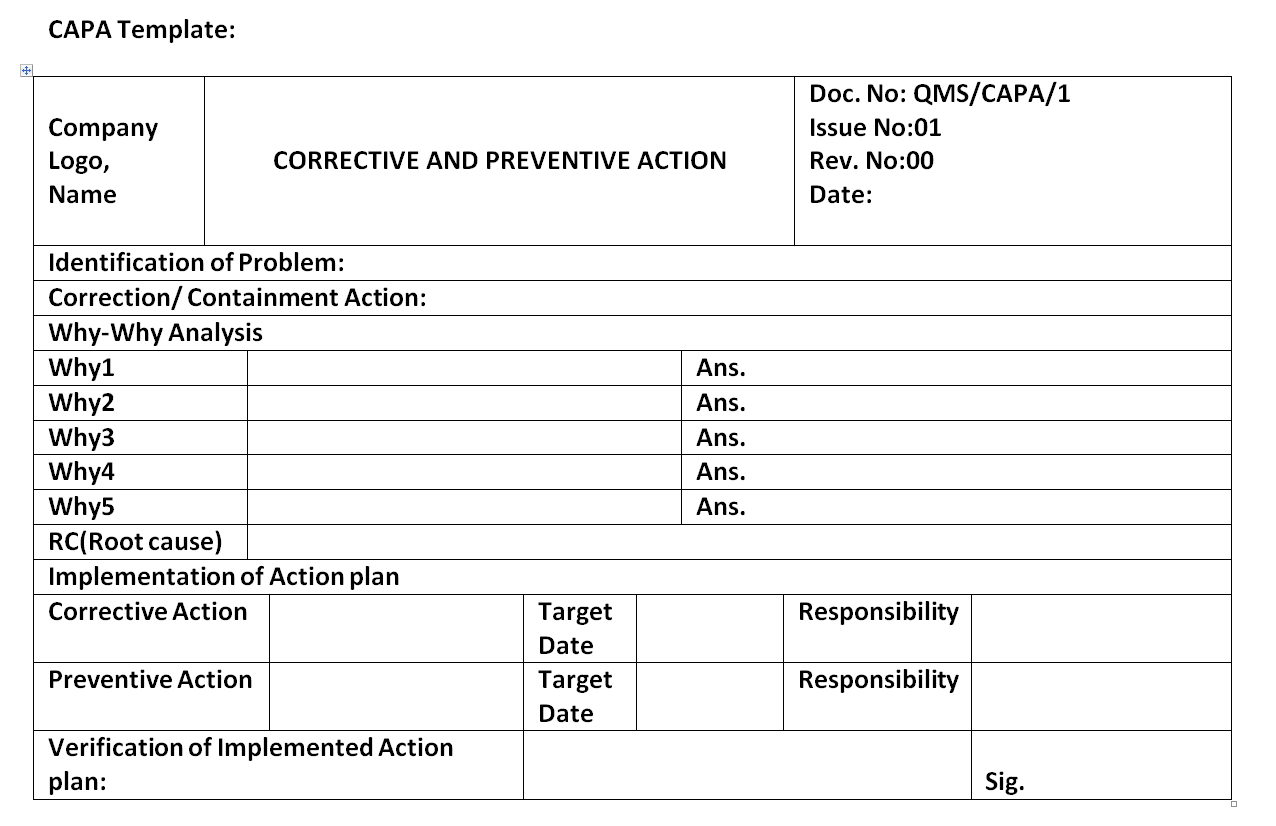
Corrective and preventive action plan CAPA report form
Corrective and Preventive Action Format CAPA with Example Download
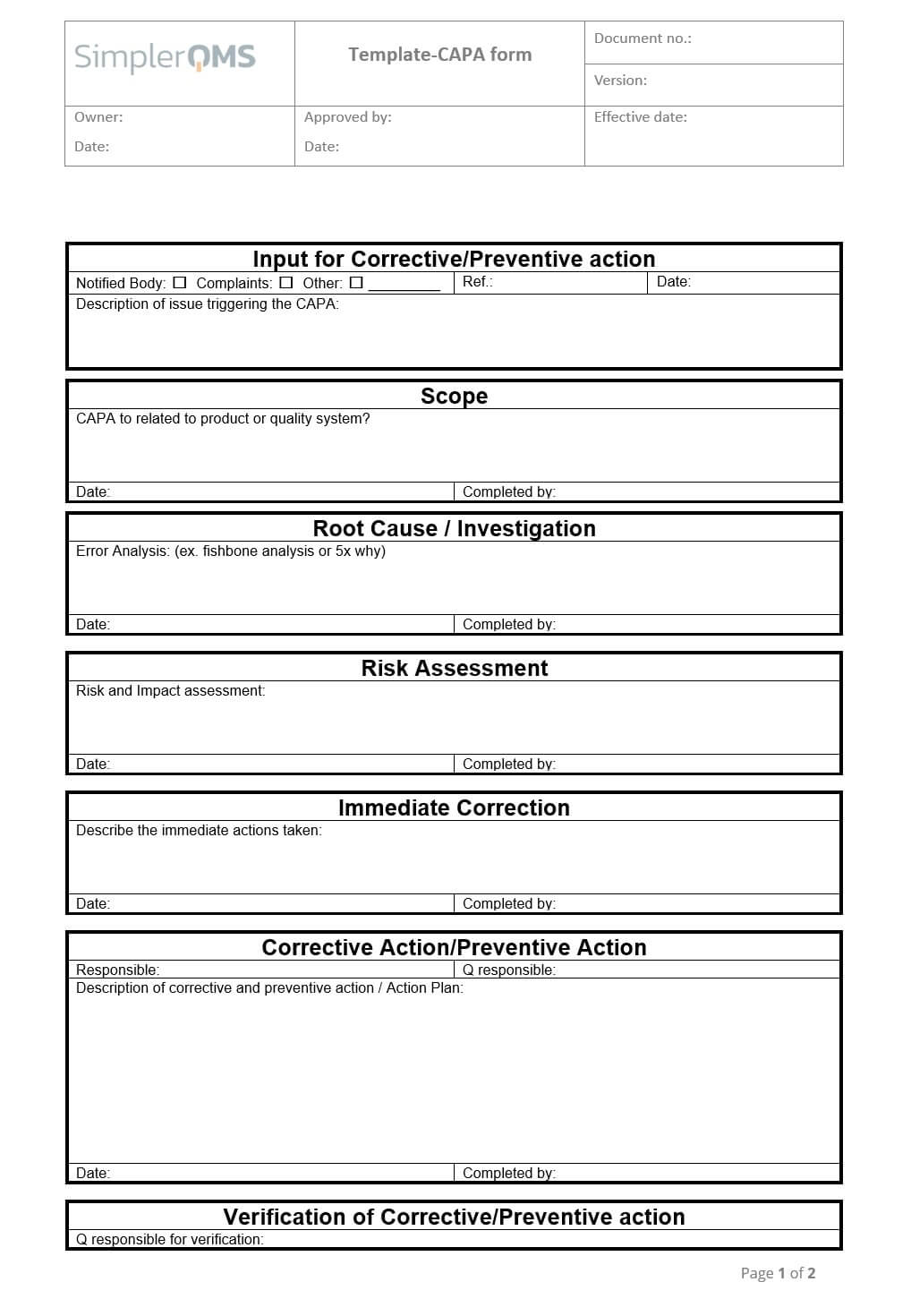
Capa Form Template Free Printable Form, Templates and Letter
CAPA form Corrective action and preventive action
Capa Form Template Free
Sample Capa form Beautiful Corrective Action Report Example Action
Corrective and preventive action plan CAPA report form
Capa Form Template Free Printable Templates
A Very Important Tool During The Capa Process Is The Capa Form, Especially In Highly Regulated Life Science Industries.
The Report Must Begin With A Statement Of The.
Web Capa(Corrective And Preventative Action) Management Is The Most Crucial Component Of A Strong And Compliant Quality Management System.
Usual Details Include Where The Problem.
Related Post: